
Robotic bronchial sleeve left upper lobectomy
Introduction
Parenchymal-preserving lung resection is becoming increasingly important with improved lung cancer screening and diagnosis of earlier and smaller lung cancers (1-3). The importance of lung preservation in pulmonary surgery has been recognized for over 50 years since the first bronchial resections and reconstructions were published (4,5). With the advancement of minimally invasive techniques for lung resection over the last few decades, there is increasing opportunity and need to perform parenchymal-sparing procedures with minimal morbidity. Sleeve lobectomy is a well-described procedure that aims to achieve lobectomy but avoid the morbidity of pneumonectomy. While this is often performed via thoracotomy, there are several reports of sleeve lobectomy being performed through a video-assisted thoracoscopic surgery approach, and more recently through robotic techniques (6-8).
The left upper lobe sleeve lobectomy is a technically challenging and potentially hazardous operation due to the potential need to control the proximal left main pulmonary artery. The main pulmonary artery appears in the left pleural space under the aortic arch, and both are close to the recurrent laryngeal and phrenic nerves. In addition, the branches of the pulmonary artery in this location are short and fragile, and bleeding from a tear or avulsion here can be difficult to control due to the challenges of working under the aortic arch and due to tethering from the ligamentum arteriosum. In addition, at least in adults, in our experience it is more common for patients to require a double sleeve (resection and reconstruction of both the artery and bronchus) due to tumor involvement of the pulmonary artery. Less common is the need for a bronchial sleeve without arterial sleeve, such as in the setting of an endobronchial carcinoid tumor. A tear of any one of the proximal pulmonary artery branches typically leads to potentially massive bleeding and subsequent conversion to thoracotomy. Therefore, a minimally invasive approach should be performed in select patients with caution by an experienced surgeon. However, with modern high definition cameras coupled with the improved angles, ergonomics, and increased dexterity offered by robotic arms and instruments, robotic sleeve lobectomy of the left upper lobe can be performed safely through a robotic approach (9,10).
Surgical technique
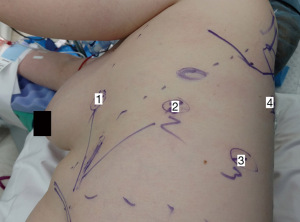
For our sleeve left upper lobectomy cases, we perform a completely portal robotic lobectomy (Video 1) using four robotic arms on the Da Vinci Xi robotic system (Intuitive Surgical, Sunnyvale, CA, USA). The patient is positioned in the right lateral decubitus position. We start with an 8 mm robotic port in the 7th intercostal space along the mid axillary line. Two additional 8 mm ports are placed approximately four fingerbreadths away anteriorly and posteriorly, and an additional 8 mm port is placed posteriorly. We use a 15 mm Airseal® System (ConMed, Utica, NY, USA) insufflation port in the 8th intercostal space at the anterior axillary line. The camera goes through arm 2. In arm 1 we use a fenestrated bipolar grasper or Cadiere forceps, in arm 3 we use a monopolar hook electrocautery (although some surgeons prefer to use bipolar electrocautery with a long dissector), and in arm 4 we use a tip-up fenestrated grasper for retraction (Figure 1). For stapling, we previously utilized hand-held staplers through the assistant port, either the Ethicon Echelon™ stapler (Ethicon, Somerville, NJ, USA) or the Covidien Endo GIA™ stapler (Medtronic, Dublin, Ireland). We felt that, in the hands of a skilled bedside assistant, hand-held staplers were safer than the more cumbersome first-generation robotic staplers. As we gained experience with the newer generation of robotic staplers, we now prefer to use these since the robotic stapler affords the surgeon with complete control over the instrument. When utilizing the robotic staplers, we upsize the arm 1 port as, this is the most anterior port with the largest rib spaces.
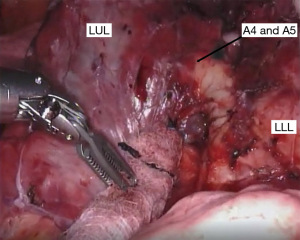
If the fissure is fairly complete, we begin our dissection in this space to expose the pulmonary artery. Removing the inter- and intra-lobar lymph nodes aids in the dissection since these nodes are usually found at arterial branch points. The dissection can be continued more proximally to identify branches of the pulmonary artery to the left upper lobe (Figure 2). At this point, the remaining fissure can be opened inferiorly with a linear stapler from the assistant port. This allows better visualization of the arterial branches going to the lingula (Figure 3), which can be divided with a vascular linear stapler.
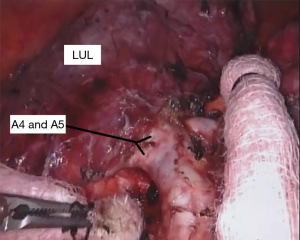
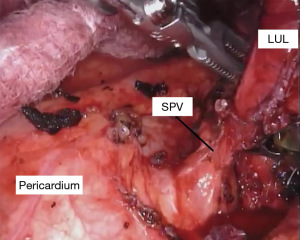
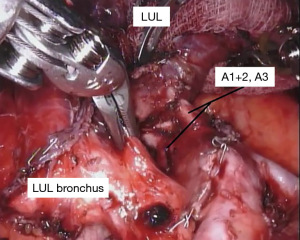
With the fissure opened and the arterial branches to the lingula divided, the posterior and apical segmental arteries can be dissected (Figure 4) and divided. Retracting the left upper lobe cephalad and posteriorly, the superior pulmonary vein can be encircled and divided (Figure 5). At this point, the apicoposterior (A1+2) and anterior (A3) segmental arterial branches can be better identified (Figure 6) and divided. This can be accomplished from either an anterior approach or from the fissure, whichever provides the best exposure and angle for stapling.
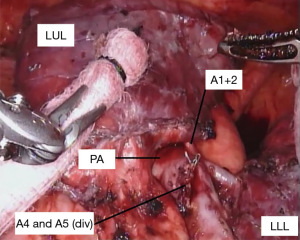
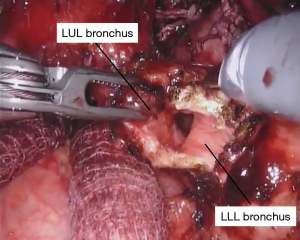
When only a bronchial sleeve is required, we first divide all of the arterial branches to the left upper lobe. After performing a confirmatory intra-operative bronchoscopy, we sharply transect the lower lobe bronchus. The left mainstem bronchus is divided just proximal the takeoff of the left upper lobe (Figure 7), according to the location of the pathology. Stay sutures (2-0 silk) are placed on the left mainstem bronchus. Additional stay sutures can be placed on the lower lobe bronchus. We limit our dissection of the bronchus to just the area of division to try to preserve as much of the blood supply as possible. It is important to take care when dividing the left upper lobe bronchus to not leave too much of the secondary carina cartilage adjacent to the membranous airway, as this can be a cause of obstruction of the anastomosis.
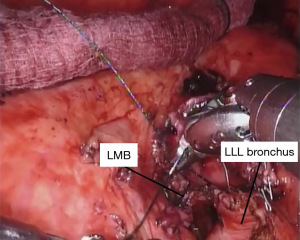
Once we confirm that our margins are negative, we perform our anastomosis with a running 4-0 PDS™ (Ethicon) suture starting posteriorly and working around anteriorly. We have also had success using 3-0 V-loc™ (Covidien) sutures starting laterally on either side and then working towards the center anteriorly (Figure 8). It is important to pay attention to the orientation of the airway as well as size mismatch. We have not had any anastomotic strictures with either suture when used in a running fashion and the majority of bronchial sleeve left upper lobectomies we have performed have been in children. The type of suture used is mainly based on surgeon preference—although we have found that V-loc suture is somewhat easier to manage since it does not slip once passed through the tissue. Care must be taken when performing the anastomosis to not occlude the superior segmental bronchus. After the anastomosis between the left lower lobe and the left mainstem bronchus is complete, the suture is tied and cut. We inflate the left lung to verify there is no air leak under instilled saline. To protect the anastomosis, we harvest a vascularized pedicle of pericardial fat, thymus, or an intercostal muscle flap. Typically, pericardial fat combined with the thymus is readily available and reaches well. We recommend tacking the flap completely around the anastomosis.
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and the accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Comments
Anatomical planning and considerations
The suitability of sleeve lobectomy is ultimately confirmed at the time of surgery. Therefore prior to performing sleeve lobectomy, the surgeon should be prepared to perform a pneumonectomy if the patient will tolerate it. In addition, even suitable candidates may require pneumonectomy in the setting of bleeding or other complications during the sleeve resection.
Sleeve lobectomy is only feasible when the bronchial and vascular structures to the remaining lobes or segments are free of disease. A preoperative bronchoscopy should be performed and carefully reviewed in conjunction with the imaging. Biopsies at the site of planned anastomosis can also help ensure a safe margin can be achieved with sleeve resection.
The left upper lobe has significant variation in the anatomy of the pulmonary artery branches. Careful review of a preoperative computed tomography (CT) scan should be performed to help plan and anticipate the patient’s anatomy during dissection. A three-dimensional reconstruction of the pulmonary anatomy can be particularly helpful to delineate the relationship of the arterial branches to the bronchus and veins (11,12).
If planning to perform mediastinoscopy as part of staging, it can be helpful to do this no more than 1–2 days before the sleeve is planned or at the time of surgery, as the dissection mobilizes the left mainstem bronchus and may potentially decrease tension on the anastomosis. Moreover, as for any left pulmonary resection, performing a mediastinal lymph node dissection first in the operation helps facilitate the procedure by exposing the vascular anatomy. If mediastinoscopy is needed, we prefer to perform this on the day of the procedure.
Technical considerations and potential pitfalls
As noted previously, the left upper lobe has treacherous variability in its arterial supply and careful identification of all the branches of the pulmonary artery to the upper lobe is crucial and the surgeon must remain vigilant of any unidentified arterial branches.
The proximity to the aorta makes this dissection particularly hazardous and the surgeon should always keep this structure in sight or understands the relationship to the structures being dissected. Proximal tumors with bulky disease in the window at the origin of the pulmonary artery can make access to the pulmonary artery difficult. In this case, division of the adventitia and pleura that reflect off the aorta toward the pulmonary artery and mobilization of the mass inferiorly can help improve exposure. In addition, manipulation of a bulky tumor can torque the pulmonary artery and cause a tear or avulsion. Two maneuvers can give additional mobility in this setting; opening the pericardium; and dividing the remnant of the ligamentum arteriosum.
The recurrent nerve can also be injured during dissection of the pulmonary artery and during lymphadenectomy. To avoid this, identify the vagus nerve as it courses over the midpoint of the aorta. If any involved nodes appear to involve the vagus nerve, it can be divided close to the pulmonary artery and the nodes carefully dissected away from the underside of the aorta, preserving the recurrent nerve.
Another common scenario is an incomplete fissure. In this case, we start by dissecting, encircling, and dividing the superior pulmonary vein first. The arterial branches can then be dissected from both the hilar approach as well as from the fissure.
Typically, after a left upper lobe sleeve lobectomy, the ongoing pulmonary artery is not excessively long relative to the airway and therefore does not end up kinked. This can be assessed by pulling the lower lobe bronchus up to the mainstem bronchus prior to carrying out the anastomosis. If there is a significant concern that the ongoing pulmonary artery is too long and is kinked as a result, a double sleeve may be required with resection of a portion of the pulmonary artery and subsequent anastomosis. However typically the intervening thymic flap puts additional tension on the artery so that in our experience it does not end up appearing too long or kinking.
Operative complications for a robotic left upper lobe sleeve lobectomy parallel those of an open approach. Given the relative infrequency of these operations, there is not enough experience at this time to definitively compare complications between the two approaches for the left upper lobe sleeve lobectomy. At our institution, we report an estimated blood loss for this procedure to be routinely under 100–150 mL for this procedure. Anecdotally, we believe this is consistent with an overall decrease in total blood loss and need for transfusion compared to open procedures. Post-operatively, we ensure that patients participate in early ambulation and aggressive pulmonary toileting, including use of scheduled nebulizers to loosen secretions for pneumonia prevention. Performing a robotic sleeve lobectomy typically adds approximately 60–90 minutes of operative time, which includes the added dissection required, the robotic anastomosis, and flap coverage. However, some of the added time with the robotic approach may counter additional time spent achieving hemostasis and chest closure via the open approach.
Finally, if the lung does not reinflate on two lung ventilation, a bronchoscopy should be performed to assess the anastomosis. Occasionally, the anastomosis may need to be revised if there is a technical issue inhibiting adequate ventilation and lung expansion. Also, as robotic surgery is typically performed with insufflated CO2, one must remember to “de-pressurize” the chest prior to attempting to expand the lower lobe.
Acknowledgments
We would like to thank Dr. Lawrence Glassman (Department of Cardiovascular and Thoracic Surgery, Northwell Health, New York, USA) and Dr. Richard Lazzaro (Division of Thoracic Surgery, RWJBarnabas Health, New Jersey, USA) for providing the surgical video and allowing us to use this resource as part of this article.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Richard Lazzaro) for the series “Robotic Anatomic Pulmonary Resection” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-151/coif). The series “Robotic Anatomic Pulmonary Resection” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this manuscript and the accompanying images and video. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Macke RA, Schuchert MJ, Odell DD, et al. Parenchymal preserving anatomic resections result in less pulmonary function loss in patients with Stage I non-small cell lung cancer. J Cardiothorac Surg 2015;10:49. [Crossref] [PubMed]
- Keenan RJ, Landreneau RJ, Maley RH Jr, et al. Segmental resection spares pulmonary function in patients with stage I lung cancer. Ann Thorac Surg 2004;78:228-33; discussion 228-33. [Crossref] [PubMed]
- Okada M, Koike T, Higashiyama M, et al. Radical sublobar resection for small-sized non-small cell lung cancer: a multicenter study. J Thorac Cardiovasc Surg 2006;132:769-75. [Crossref] [PubMed]
- Paulson DL, Shaw RR. Preservation of lung tissue by means of bronchoplastic procedures. Am J Surg 1955;89:347-55. [Crossref] [PubMed]
- Paulson DL, Shaw RR. Bronchial anastomosis and bronchoplastic procedures in the interest of preservation of lung tissue. J Thorac Surg 1955;29:238-59. [Crossref] [PubMed]
- Han Y, Zhou S, Yu D, et al. Video-assisted left upper bronchial sleeve lobectomy. J Thorac Dis 2013;5:S304-6. [PubMed]
- Jiao W, Zhao Y, Wang X, et al. Video-assisted thoracoscopic left upper lobe sleeve lobectomy combined with pulmonary arterioplasty via two-port approach. J Thorac Dis 2014;6:1813-5. [PubMed]
- Onugha OI, McKenna RJ. Thoracoscopic Sleeve Resection/Bronchoplasty for Lung Cancer. In: Sugarbaker DJ, Bueno R, Burt BM, et al. (eds). Sugarbaker’s Adult Chest Surgery. 3rd ed. New York, NY: McGraw-Hill Education; 2020.
- Cerfolio RJ. Robotic sleeve lobectomy: technical details and early results. J Thorac Dis 2016;8:S223-6. [PubMed]
- Geraci TC, Ferrari-Light D, Wang S, et al. Robotic Sleeve Resection of the Airway: Outcomes and Technical Conduct Using Video Vignettes. Ann Thorac Surg 2020;110:236-40. [Crossref] [PubMed]
- Kanzaki M, Kikkawa T, Shimizu T, et al. Presurgical planning using a three-dimensional pulmonary model of the actual anatomy of patient with primary lung cancer. Thorac Cardiovasc Surg 2013;61:144-50. [Crossref] [PubMed]
- Chan EG, Landreneau JR, Schuchert MJ, et al. Preoperative (3-dimensional) computed tomography lung reconstruction before anatomic segmentectomy or lobectomy for stage I non-small cell lung cancer. J Thorac Cardiovasc Surg 2015;150:523-8. [Crossref] [PubMed]
Cite this article as: Pommerening MJ, Young JS, Marshall MB. Robotic bronchial sleeve left upper lobectomy. Curr Chall Thorac Surg 2023;5:42.


