
Robotic left lower segmentectomy operations: a focused review
Introduction
The lung cancer study group (LCSG) established the standard for anatomic resection (lobectomy) as the best surgical treatment for early-stage non-small cell lung cancer (NSCLC) (1). Although sublobar resection was associated with a 3-fold increased risk of recurrence and inferior 5-year survival, this subgroup included tumors up to 3 cm and a significant proportion of wedge resections. Since the LCSG was published, several studies have demonstrated that in select patients and for specific tumors, segmentectomy may afford an equivalent survival and recurrence risk without the need to perform a full lobectomy (2-5). In fact, most recent data suggest that the standard surgical procedure for small, peripheral stage IA NSCLC (≤2 cm, consolidation-to-tumor ratio >0.5) is segmentectomy or wedge resection with appropriate margins and lymph node dissection (6,7). Although technically more challenging than lobectomy, robotic resection affords the surgeon specific advantages that facilitate segmentectomy. An understanding of which patients are most appropriate for segmentectomy, technical considerations, and a review of potential pearls and pitfalls will help the robotic surgeon to successfully incorporate this technique into their clinical practice.
Patient selection and pre-operative work-up
The majority of patients who are eligible for video-assisted thoracoscopic surgery (VATS) are appropriate candidates for robotic segmentectomy. All patients undergo standard preoperative risk assessment that is consistent with established National Comprehensive Cancer Network (NCCN) guidelines (8). Chest computed tomography (CT) scan with intravenous contrast is strongly encouraged and particularly helpful in preparation for segmentectomy to identify both arterial and venous drainage and their relationship to adjacent structures. Three-dimensional reconstruction of imaging can further aid in delineating anatomical relationships for surgical planning. Positron emission topography (PET) scan, cardiac risk stratification including a stress test, pulmonary function tests (PFT’s), and brain MRI when indicated are also part of the standard workup of all NSCLC patients, with the exception of part solid or pure ground glass nodules. We do not specifically recommend using PFT’s alone as the determinant for the performance of segmentectomy but as a datapoint within a comprehensive assessment of patient risk for surgery.
Consideration for segmentectomy as an alternative to lobectomy should include a number of important patient specific factors. As identified in a LCSG subgroup and confirmed in other work, there are survival and recurrence advantages associated with segmentectomy over wedge resection, particularly for smaller tumors (<2 cm) (2). In these cases, the advantages of lobectomy rather than segmentectomy were less clear. These authors therefore recommended consideration for segmentectomy in patients with more limited pulmonary reserve, including cases where postoperative predicted (ppo) forced expiratory volume in one second (FEV1) and diffusion capacity (DLCO) may be lower than 35–40% predicted following lobectomy. However, recent data now demonstrate segmentectomy is similar to lobectomy with respect to disease-free and overall survival, at least in patients with ≤2 cm peripheral NSCLC and pathologically confirmed negative hilar/mediastinal lymph nodes (6,7). In patients with synchronous primary lung cancers in separate lobes, segmentectomy may confer the advantages of anatomic resection without the need for bilobectomy, which may be associated with increased risk for postoperative pulmonary complications. Segmentectomy for synchronous nodules is also preferable to a hybrid approach (resection of one nodule, radiation to another), which may likely be associated with a higher risk local recurrence compared to two segments. Surgical resection of part solid nodule or pure ground glass nodules, associated with significantly lower risk for locoregional spread compared to their solid counterparts, are also appropriate for segmental resection when technically feasible. We strongly prefer this approach to wedge resection to reduce the risk of local recurrence and to achieve a true hilar lymph node dissection. When technically possible, solid tumors <2 cm that are not associated with enlarged or PET avid lymph nodes, are appropriate for segmentectomy. These recommendations are based on best available evidence that segmentectomy for smaller tumors is associated with similar recurrence and survival data compared to lobectomy for tumors <2 cm (2-7). We do not recommend segmentectomy for tumors >2 cm as there is not sufficient data to suggest these larger tumors have similar outcomes following segmentectomy.
Although segmentectomy for solid tumors is most commonly performed for nodules located in the superior segment, the performance of basilar segmentectomy, while technically more challenging, is based on similar principles. Reference to preoperative high resolution CT imaging is helpful to determine if an adequate surgical margin can be achieved, particularly given that up to 30% of tumors may involve more than one segment (9).
Review of relevant anatomy
In-depth knowledge of lung anatomy is vital to the success of any robotic resection. Critical to the performance of robotic lung resection, the camera is placed to allow clear view of the hilum/lung from an inferior perspective. Depending on the patient’s anatomy, type of fissure (complete versus incomplete), and pleural space (presence or absence of adhesions, etc.), anterior and posterior approaches are utilized to perform a left lower segmentectomy. As such, it is necessary to understand spatial relationships within the hilum, segmental anatomy, and common anatomic variations.
The left hilum courses under the aortic arch, with the left pulmonary artery lying anterior and superior to the left mainstem bronchus. Further, the left pulmonary artery is short (compared to the right). If pulmonary artery bleeding occurs during dissection, it is often beneficial to obtain proximal control of the pulmonary artery at the left hilum. The left superior pulmonary vein is anterior and inferior to the left pulmonary artery, with the inferior pulmonary vein positioned more inferior and posterior to the superior vein. It should be noted that the superior and inferior pulmonary veins form a common trunk before entering the left atrium in about 25% of the population (10). From an anterior to posterior anatomic position, the structures encountered when approaching the left lower lobe are: pulmonary vein, pulmonary artery, and then bronchus.
A segmentectomy is the resection of the entire pulmonary segment, dividing the individual bronchus, artery, and vein of the corresponding segment. The left lower lobe has four segments: superior, anteromedial basal, lateral basal, and posterior basal. Segmental arteries usually follow the course of the bronchi and are commonly located on the superior or lateral surface of the segmental bronchi. The terminal branches of the left pulmonary artery are the superior segmental artery and the basilar artery and are found in the fissure. More than one superior segmental artery may exist. Veins run in the intersegmental planes, usually without a close association to the bronchi (but they often lie medial or inferior to the bronchi). The segmental veins vary more than arteries, and arteries vary more than the segmental bronchi (10). Unlike upper lobe segmentectomy, we generally do not aim to identify the lower lobe segmental vein anatomy at the onset of a procedure and instead focus on pulmonary artery and bronchus ligation and division, which will expose appropriate segmental venous drainage.
As with lobectomy, one must also be mindful of the left phrenic nerve, left recurrent laryngeal nerve, and esophagus during dissection in order to avoid injury of these structures.
Operative technique
General anesthesia is utilized, and one-lung ventilation is achieved via a double-lumen endotracheal tube (ETT). Bronchoscopy should be performed to confirm position of the ETT and to assess segmental anatomy. The patient is placed in the right lateral decubitus position with appropriate padding and securing of the patient.
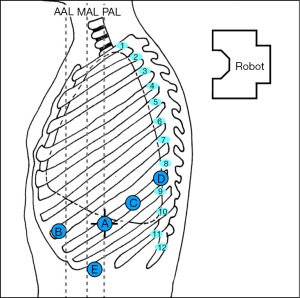
The robot is usually positioned perpendicular to the patient’s body. Figure 1 depicts our preferred configuration for ports. We begin port placement (8 mm) with an incision for the robotic camera in the 8th intercostal space (designated as robotic arm 2) about the posterior axillary line (A in Figure 1). A trick to placing the camera port is identifying the junction of the xiphoid and sternum and following that line posteriorly until the highest point of the chest is identified, this is usually at the 7th or 8th intercostal space. For lower lobe resections, the 8th intercostal space is preferred. Either a 0° or 30° robotic camera can be used, depending on surgeon preference. The 30° camera can be helpful when having to look up and over the apex of the lung/superior hilum for dissection of station 5 and 6 lymph nodes. Once the camera is inserted into the thoracic cavity, exploration ensues, ruling out advanced disease that would preclude segmentectomy. Carbon dioxide insufflation is initiated to assist with visualization (pushes diaphragm inferiorly, helps with preventing lung inflation, and decreases bleeding). Intercostal nerve blocks are performed under direct visualization to assist with post-operative pain control (we use liposomal bupivacaine). The remaining ports are then placed, including the most anterior port (12 mm, for robotic arm 1) near the anterior axillary line which will be the primary stapling port (B in Figure 1). It should be as anterior and medial as possible; it may be best to place this port one intercostal space up (7th intercostal space) so there is room for the assist port. It is also helpful to have more distance between this port and the camera port, approximately 10–12 cm, to achieve optimal stapling angles. A third incision is placed lateral/posterior to the camera port, also in the 8th intercostal space for robotic arm 3 (12 mm port; C in Figure 1). Robotic arm 4 (8 mm port) is most posterior, about 1–2 cm lateral to the spinous process of the vertebral body (D in Figure 1). The distance between the 2nd and 3rd and 3rd and 4th ports can be closer together, with a minimum distance of 8 cm. The assistant port (12 mm) is placed such that it is triangulated from robotic arms 1 and 2 and placed in the 9th or 10th intercostal space (E in Figure 1). The assistant port should be placed at the junction of the diaphragm and chest wall.
Various robotic instruments can be used; we typically use the long bipolar grasper in arm 1, cadiere forceps in arm 3, and a tip-up fenestrated grasper in arm 4.
Superior segmentectomy (S6)
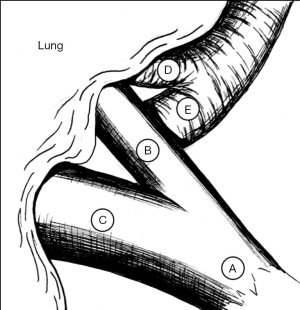
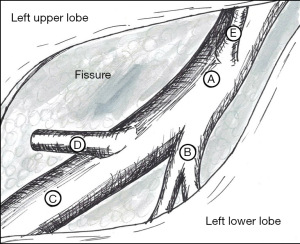
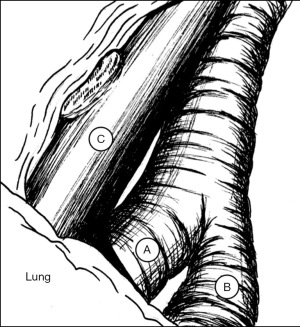
As with robotic lobectomy, thoracic lymphadenectomy is performed and should include stations 8, 9, 7, and 5/6. We perform the thoracic lymphadenectomy first, and the lymph nodes are sent for permanent analysis. Lymphadenectomy should also include stations 10, 11, and 12; this helps to define critical structures and is necessary to isolate the segmental artery, vein, and bronchus. The inferior pulmonary ligament is divided, and during dissection any station 8 and 9 lymph nodes encountered are resected. The inferior pulmonary vein is cleared anteriorly and posteriorly. Dissection is carried along the posterior hilum, opening of the pleura while the lung is retracted anteriorly. Using a cigar roll is helpful for retraction (pushing the lung parenchyma, as opposed to grabbing the lung itself). Distal dissection of the inferior pulmonary vein can be carried out until the superior segmental pulmonary vein is identified (B in Figure 2) to facilitate circumferential dissection. A vessel loop can be placed to mark the segmental vein for division later. Lymph node station 7 is then dissected out. Next, identification of the superior segment pulmonary artery branch is achieved by positioning the lobe back into anatomic position and opening the major fissure (B in Figure 3). Completing the posterior aspect of the fissure with a stapler (blue load) aides in visualization and additional dissection. Lymph nodes are often encountered during dissection, and usually mark branch points. Once the superior segmental pulmonary artery branch is circumferentially dissected, it can be divided with a curved tip vascular staple load (white). If too bulky, one can also use a vessel sealer ± medium robotic clips to divide the arterial branch. Returning to the fissure and performing additional dissection allows visualization of the superior segmental bronchus (A in Figure 4). Alternating between an anterior and posterior approach is helpful for dissection and appropriate identification of the segmental bronchus. Once circumferentially cleared, the segmental bronchus is divided using a blue staple load. The superior segmental vein is then divided in a similar fashion. The final step is division of the parenchyma with thick tissue staplers (green or blue). This can be achieved by administration of indocyanine green (ICG) and use fluorescence imaging (FireFly) or based on changes in parenchymal perfusion. Having two 12 mm ports (arms 1 and 3) is helpful, allowing two different angles for division of these structures. To complete the lymphadenectomy, stations 5/6 are assessed by positioning left upper lobe inferiorly visualizing the superior aspect of the hilum, and any lymph nodes present are resected being careful to avoid recurrent laryngeal nerve injury.
Basilar segmentectomy [superior segment sparing lobectomy (S8 + S9 + S10)]
The steps of a basilar segmentectomy are the same as the superior segmentectomy, except the respective common basal venous trunk, basal pulmonary arterial trunk, and common basal bronchus are divided. The inferior pulmonary vein is cleared and dissected distally. The common basal segmental vein is the inferior, broader branch (C in Figure 2). The basal arterial trunk is also identified in the fissure (C in Figure 3). Once the vasculature to the basilar segments are divided, the common basilar bronchus is circumferentially dissected (B in Figure 4) and divided. The parenchyma is divided using similar techniques as previously described.
Individual basilar segmentectomy (S8, S9, or S10)
Individual basilar segmentectomy [anteromedial basal (S8), lateral basal (S9), or posterior basal segmentectomy (S10)] is feasible, but also more technically challenging. Identification of appropriate pulmonary artery and vein branches begins by reviewing a high resolution preoperative contrasted CT scan. The same principles and steps are followed as described above, except dissection is carried more distally from the hilum to identify each respective segmental pulmonary vein, pulmonary artery, and bronchus and divided accordingly. We generally recommend exposure of the entire pulmonary artery including branches to S8, S9, and S10 to avoid inadvertent division of the wrong branch. After division of the pulmonary artery branch, the respective bronchus is identified behind it, allowing for circumferential mobilization and division. Jet insufflation via bronchoscopy may allow for better definition of the segmental anatomy. The segmental venous branch is typically identified behind the bronchus, although there is some variability in its location depending on the segment to be excised. In select cases where bronchial anatomy is not clear, we recommend intraoperative bronchoscopy with transillumination to confirm the appropriate segmental division. Parenchymal division can be performed either with the use of ICG or identifying the border of perfused lung as described previously. Knowledge of the segmental anatomy significantly impacts the technical success associated with performing these more complex sublobar resections.
Ethical consideration
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Robotic segmentectomy is a useful technique to manage early-stage NSCLC. Increased robotic adoption has resulted in a concurrent increase in the performance of a true segmentectomy (division of artery, vein, and bronchus to the segment rather than pulmonary artery division and a large wedge). Even for the advanced robotic lung resection, segmentectomy other than superior segments or lingular sparing left upper lobes require additional technical consideration and a more in depth understanding of the relevant anatomy compared to lobectomy. 3D CT scan reconstruction with intravenous contrast may be helpful to understand the spatial relationships that are important to complete these more technically challenging resections. Superior segmentectomy of the left lower lobe may itself be difficult based on the angle of takeoff of the pulmonary artery, the need to staple most commonly with the left hand, and the proximity of this branch to the aorta, which may require use of vascular clips and/or energy ligation. An understanding of segmental anatomy, thorough resection of regional lymph nodes, and careful exposure of all critical structures in the vicinity of a tumor help to facilitate a safe and effective procedure.
Conclusions
A shift to minimally invasive lung resection has occurred over the last two decades in an effort to decrease morbidity associated with thoracotomy. The robotic platform offers advantages over the VATS approach, including improved visualization (3D) and wristed instrumentation. While lobectomy remains the gold standard treatment for early-stage lung cancer, certain patients and tumor subtypes can be managed with segmental resection with improved postoperative pulmonary reserve and what appears to be similar oncologic outcomes. As such, robotic segmentectomy may be an appropriate treatment option in these patients. While technically more challenging than a lobectomy, segmentectomy remains an important skillset in the toolbox of a thoracic surgeon.
Acknowledgments
The authors would like to thank Christine Rasmussen, M.Ed. (www.Christyrasmussenart.com) for her medical illustrations.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Richard Lazzaro) for the series “Robotic Anatomic Pulmonary Resection” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-149/coif). The series “Robotic Anatomic Pulmonary Resection” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patients for publication of this manuscript. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg 1995;60:615-22; discussion 622-3. [Crossref] [PubMed]
- Altorki NK, Yip R, Hanaoka T, et al. Sublobar resection is equivalent to lobectomy for clinical stage 1A lung cancer in solid nodules. J Thorac Cardiovasc Surg 2014;147:754-62; Discussion 762-4. [Crossref] [PubMed]
- Campione A, Ligabue T, Luzzi L, et al. Comparison between segmentectomy and larger resection of stage IA non-small cell lung carcinoma. J Cardiovasc Surg (Torino) 2004;45:67-70. [PubMed]
- El-Sherif A, Gooding WE, Santos R, et al. Outcomes of sublobar resection versus lobectomy for stage I non-small cell lung cancer: a 13-year analysis. Ann Thorac Surg 2006;82:408-15; discussion 415-6. [Crossref] [PubMed]
- Kilic A, Schuchert MJ, Pettiford BL, et al. Anatomic segmentectomy for stage I non-small cell lung cancer in the elderly. Ann Thorac Surg 2009;87:1662-6; discussion 1667-8. [Crossref] [PubMed]
- Altorki N, Wang X, Kozono D, et al. Lobar or Sublobar Resection for Peripheral Stage IA Non-Small-Cell Lung Cancer. N Engl J Med 2023;388:489-98. [Crossref] [PubMed]
- Saji H, Okada M, Tsuboi M, et al. Segmentectomy versus lobectomy in small-sized peripheral non-small-cell lung cancer (JCOG0802/WJOG4607L): a multicentre, open-label, phase 3, randomised, controlled, non-inferiority trial. Lancet 2022;399:1607-17. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Non-Small Cell Lung Cancer. Version 3.203 - April 13, 2023. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1450
- Horinouchi H, Nomori H, Nakayama T, et al. How many pathological T1N0M0 non-small cell lung cancers can be completely resected in one segment? Special reference to high-resolution computed tomography findings. Surg Today 2011;41:1062-6. [Crossref] [PubMed]
- Chin CS, Shahani R. Anatomy of the Thorax. In: Sellke FW, del Nido PJ, Swanson SJ (eds). Sabiston & Spencer Surgery of the Chest. 9th Edition. Philadelphia: Elsevier; 2016:3-25.
Cite this article as: Eilers AL, Blasberg JD. Robotic left lower segmentectomy operations: a focused review. Curr Chall Thorac Surg 2023;5:43.


