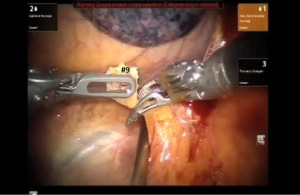
Robotic sleeve lower lobectomy
Introduction
Sleeve lobectomy was first introduced by Allison and was originally designed as an alternative to a pneumonectomy in patients with impaired pulmonary function (1). Since Allison’s report in 1954, many studies have shown that sleeve lobectomy is comparable to a pneumonectomy in terms of oncologic efficacy, morbidity, mortality, and long-term survival (2-5). In addition, presumably by preserving lung function and decreasing the complications that are inherent to a pneumonectomy, sleeve lobectomy has been shown to be superior to a pneumonectomy in terms of complications and quality of life. Sleeve lobectomy is indicated for tumors that extend past the bronchial orifice of the lobe. Although a sleeve lobectomy should be considered in all patients who would otherwise require a pneumonectomy due to the location of the tumor in the bronchus, it is especially indicated in patients with a limited respiratory reserve, patients with comorbidities, and the elderly. Presently, the indications for sleeve lobectomy are: (I) R0 achievement; (II) N0 and selected N1 disease; (III) less aggressive tumors such as carcinoids, localized squamous cell carcinoma, and selected patients with adenocarcinoma; (IV) poor lung function; (V) avoidance of pneumonectomy; and (VI) advanced age and/or comorbid conditions in presence of less aggressive disease.
Minimally invasive sleeve lobectomy has been performed by video-assisted thoracic surgery (VATS) (6). However, sleeve lobectomy by VATS has been hampered by the limitation of the 2-dimensional visualization of the operative field and the shortcomings of the conventional un-wristed instruments. The main difficulty in performing sleeve lobectomy by VATS has been the bronchial anastomosis (7).
Robotic lobectomy is now widely accepted as feasible and safe, and a number of studies have shown decreased blood loss and length of stay (8). A meta-analysis comparing robot-assisted thoracic surgery (RATS) to VATS and open approaches found RATS to be superior with respect to transfusions, complications, length of inpatient stay and 30-day mortality (9). The number of robotic lobectomies has increased in recent years (10).
In 2006, Ishikawa et al. described the technique of robotic bronchoplasty in a cadaver model (11). The robotic surgical platform by virtue of magnified 3-dimensional visualization, ease of preparation of the bronchial stumps and setup of the anastomosis, and wristed instrument maneuverability in a confined space, enables sleeve lobectomy using minimally invasive techniques (12-14). Durand reported 486 major robotic anatomic lung resections (15). Ten patients (2%) underwent robotic bronchial sleeve resections. The mean operative time was 164±43 minutes and there were no conversions to a thoracotomy.
This article outlines the technical aspects of performing a robotic sleeve lower lobectomy.
Surgical technique
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Sleeve lower lobectomy is an extension of the robotic lower lobectomy technique. We use the completely portal robotic lobectomy technique using four arms (CPRS-4) (16-19).
Sleeve right lower lobectomy
A double lumen tube is placed, and the patient is positioned in a full lateral decubitus position. The right arm is placed over pillows and positioned high enough such that access to the 4th intercostal space in the anterior axillary line is readily attained. The table is flexed in order to move the hip down and to open the intercostal spaces. The lung is deflated and placed on suction. The position of the double lumen tube is rechecked after the patient is prepped and draped.
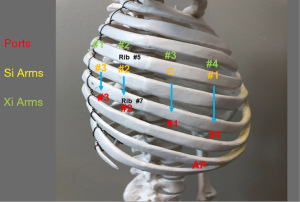
Figure 1 shows port placement with the Da Vinci Xi Platform (Intuitive Surgical, Mountainview, CA, USA).
All ports are placed in the 7th intercostal space. Ports are placed 9 cm apart.
Port #1 (8 mm) is the camera port. A line is drawn from the tip of the scapula to the costal arch. This delineates the highest point in the chest and the mid-scapular line (posterior axillary line). Pleural entry is with a Hassan needle. Saline is infused and care is taken to look for easy egress of the saline from the needle. If there is any question of pleural adhesions, we use a Visiport Instrument (Covidien Inc., Norwalk, CT, USA) for entry into the pleural space under direct vision. If the Visiport is used, a purse string is placed in the muscle layer and tied around the robot camera port in order to prevent CO2 leakage.
Warm, humidified CO2 is insufflated through this port at a flow of 6 L/min to a pressure of 6–8 mmHg in order to push the lung and diaphragm away. The other ports are placed under direct vision. Da Vinci arm #3 is placed through Port #1.
Port #2 (8 mm) is placed in the 7th intercostal space in the poster scapular line. This Port is 9 cm posterior to Port #1 and accommodates Da Vinci arm #2.
Prior to the placement of Port #3 (12 mm), a 21-gauge needle is inserted into the 7th intercostal space at costovertebral junction from the patient’s back and injects a 10 mL subpleural bubble of 0.25% bupivacaine with epinephrine near the intercostal nerve. Next, Port #3 is placed 10 cm posterior to Port #2 and 4 cm from the spine in the 7th intercostal space. This port accommodates Da Vinci arm #1. This 12 mm port allows for the use of the robotic stapler. In order to use other instruments, a 12 to 8 mm Cannula Reducer is used.
Port #4 (12 mm) is placed 9 cm anterior to Port #1 in the 7th intercostal space at the anterior scapular line. This port accommodates Da Vinci arm #1. This 12 mm port allows for the use of the robotic stapler. In order to use other instruments, a 12 to 8 mm Cannula Reducer is used.
The Assistant Port #5 uses a 10–12 Versiport trocar and is placed in the 9th intercostal space and is triangulated between Port #1 and #4. It should be two or three ribs lower than and as distant to the Da Vinci ports as possible to maximize assistant workspace. Keeping this port off the trajectory lines for those ports will facilitate the patient-side assistant’s access for retraction and other maneuvers.
Instruments include 0° and/or 30° down viewing endoscope, 5 mm Thoracic Grasper (left ③), Cadiere Forceps (left ②) and Curved Bipolar Dissector (right ①). For the anastomosis we use the small robotic needle driver (right ①) and the robotic DeBakey forceps (left ②).
The DA Vinci Xi System Endowrist staplers are used. The Endowrist Stapler 30 with White Load is used for dividing the pulmonary artery and vein. The Endowrist Stapler 45 with Green Load is used for dividing the lung tissue.
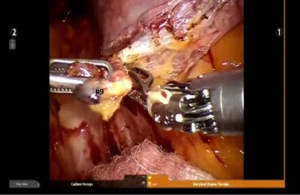
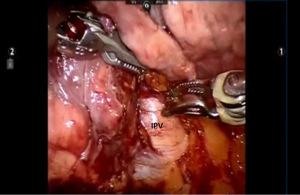
Begin by dividing the inferior pulmonary ligament and remove station #9, and #8 nodes. Dissection is carried up to the inferior border of the inferior pulmonary vein (IPV) (Figures 2,3). The most posterior arm is used to retract the lower lobe medially and anteriorly in order to remove lymph nodes from station #7. Next open the pleura anterior to the vagus nerve and divide the anterior branch of the nerve which traverses the subcarinal space. Be certain that the nasogastric tube has been removed and the esophagus is decompressed. This opens the subcarinal space and allows for better access to the station #7 nodes (Figure 4). Identify the right mainstem bronchus and stay posterior to the edge of the cartilage. Remove the station #7 nodes and control the subcarinal artery at the carina. At the end of the dissection the right and left mainstem bronchi should be visible, and the posterior aspect of the pericardium should be cleaned and clearly visible. Next, the most posterior arm is used to retract the upper lobe inferiorly during dissection of stations 2R and 4R, clearing the space between the superior vena cava (SVC) anteriorly, the trachea posteriorly, and the azygos vein inferiorly (Figure 5).
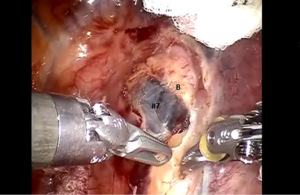
Completion of the lymph node dissection opens the mediastinal space and facilitates the dissection of the artery and the bronchus.
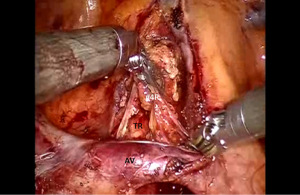
Next the lung is retracted posteriorly and held in place with the robot arm. The bifurcation of the right superior and IPVs is dissected and delineated. The location of the right middle lobar vein should be positively identified to avoid inadvertent transection. The IPV is encircled using the Cadiere Forceps and divided using a white vascular cartridge.
The anterior branch of the lower lobe pulmonary artery is most superficial and usually does not have overlying nodal tissue. This branch is identified and traced back to the main trunk of the pulmonary artery. Next the sub adventitial plane overlying the pulmonary artery is developed and nodal tissue (station #11) is removed. Retraction is released and the lung is allowed to remain in its normal position, thereby facilitating visualization of the oblique fissure. The dissection is carried posteriorly in the sub adventitial layer and the superior segmental branch of the lower lobe pulmonary artery is identified. The major fissure is then divided from an anterior to posterior direction using a stapler which is introduced from the anterior port.
The pulmonary artery branch to the lower lobe is identified. At times the superior segmental pulmonary artery and the inferior lower lobe segmental artery can be taken by encircling the pulmonary artery proximal to the takeoff of the superior segmental artery. Other times these branches need to be taken separately. We prefer to take the inferior segmental artery first, thereby making the encirclement of the superior segmental artery easier. Next the posterior aspect of the oblique fissure is divided using a stapler with a purple cartridge.
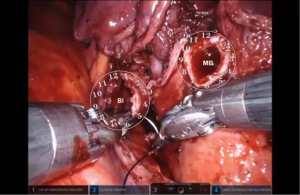
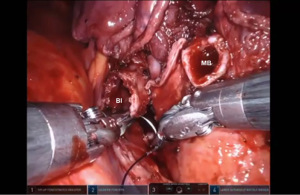
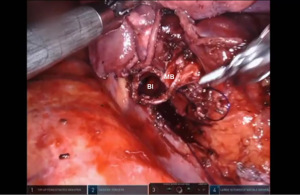
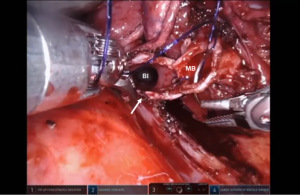
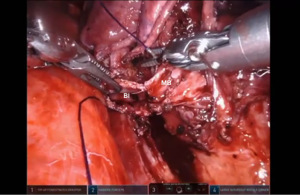
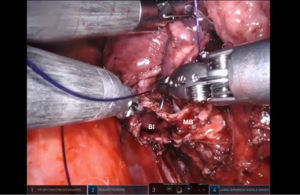
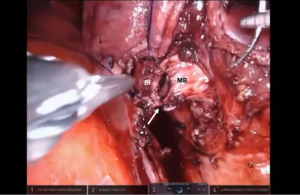
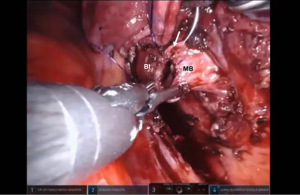
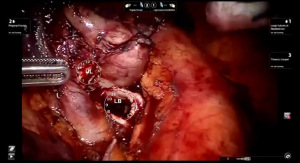
Bronchotomy is localized by using bronchoscopic guidance. The monopolar shears are used to cut the bronchus and to prepare the ends of the proximal bronchus intermedius (BI) and middle lobe bronchus (MB). The bronchi are dissected and freed extensively in order to prevent any tension at the anastomosis (Figure 6). Negative bronchial margins are confirmed by frozen section prior to the anastomosis. We have used 3-0 Vicryl, 4-0 Vicryl, and 3-0 and 4-0 V-Loc (Covidien Inc.) suture cut to an 8 cm length on an RB1 needle for the anastomosis.
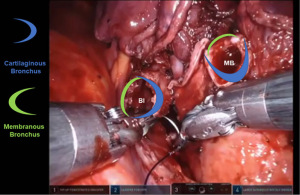
Video of the anastamosis can be accessed on https://youtu.be/zHjDGvu0lrw.
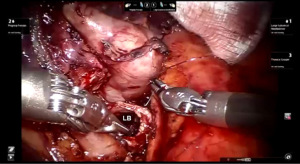
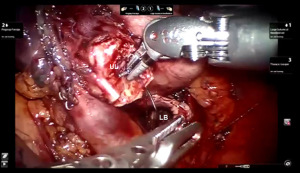
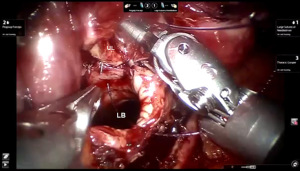
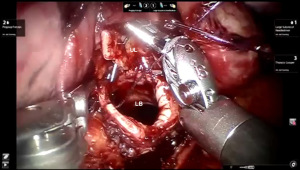
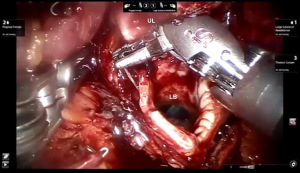
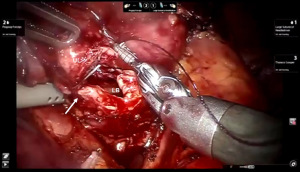
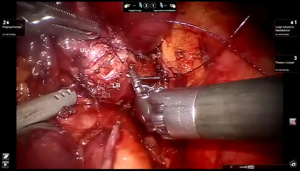
Presently we prefer a 3-0 STRATAFIX Spiral PDS Plus (Ethicon Inc., Bridgewater, NJ, USA) on an RB1 needle. The design of the STRATAFIX suture requires a single knot to be placed at the completion of the anastomosis. Anastomosis is begun with STRATAFIX #1 (S1). The S1 needle is passed from the outside in at the 5 o’clock position on BI and inside out at the and 7 o’clock position on MB (Figures 7,8). The Suture is passed through the “eyelet” and fixed. For the second bite, the S1 needle is passed outside in at the 6 o’clock position on MB and inside out at the 6 o’clock position on BI (Figure 9). Suturing with S1 is continued in a clockwise manner until the border between the cartilaginous and membranous portions of the bronchi (Figures 10,11). Next a second STRATAFIX (S2) suture is passed from outside in at the 8 o’clock position of the MB and inside out at the 4 o’clock position of the BI (Figure 12). S2 is passed through the “eyelet” and fixed. Next, S2 is used to reapproximate BI to MB in an anti-clockwise direction making sure to prevent any torsion at the anastomosis and to match up the cartilaginous walls of the bronchi (Figure 13). Suturing with S1 stops at the inferior junction of the cartilaginous and membranous walls of the bronchi (Figure 14). Once S2 reaches the superior junction of the cartilaginous and membranous walls of the bronchi, it is used to reapproximate the membranous portion of BI and MB (Figures 15,16) S1 and S2 are tied at the inferior junction of the cartilaginous and membranous walls of the BI and MB bronchi (Figure 17). The placing the knot on the cartilaginous portion of the bronchi prevents inadvertent narrowing of the membranous portion of the bronchi. After the completion of the anastomosis, CO2 is turned off, the anastomosis is submerged in warm water and tested for leaks by inflating the lung. In addition, the integrity of the anastomosis is confirmed by bronchoscopy. A 28 French chest tube is placed through the Assistant Port and positioned posteriorly in the pleural space.

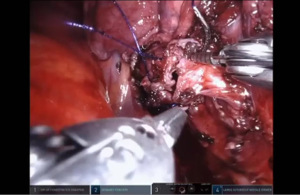
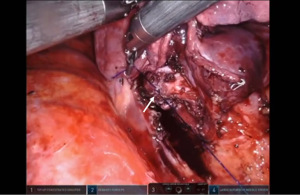
Sleeve left lower lobectomy
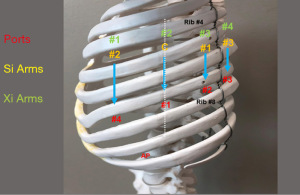
Port placement: Figures 18,19 show left sided port placement. Port placement is similar to the right sided approach.

Begin by dividing the inferior pulmonary ligament and remove station #9, and #8 nodes (Figure 20) The lung is retracted medially and anteriorly in order to remove lymph nodes from station #7. The nasogastric tube should be removed in order to create a greater space for the subcarinal and mediastinal dissection. Next open the pleura anterior to the vagus nerve. Identify the left mainstem bronchus and stay inferior to the edge of the cartilage. The station #7 nodal bundle is accessed between the IPV and the left mainstem bronchus. The nodal bundle is traced to the carina and is then removed (Figure 21). Next the upper lobe and lower lobe are retracted in opposite directions and the fissure is identified. Dissection of nodal bundle in station #11 allows for the identification of the pulmonary artery in the fissure. The artery is most superficial at the junction of the lingula, upper lobe and the lower lobe. The sub-adventitial plane is entered, and dissection is carried posteriorly under the pulmonary parenchyma in the posterior aspect of the fissure toward the main pulmonary artery. The Cadiere Forceps is used to pass a vessel loop under the pulmonary parenchyma in the posterior aspect of the fissure. A stapler with a blue cartridge is used to divide the tissue in the posterior aspect of the fissure. The sub-adventitial plane is then developed anteriorly in order to identify the lower lobe branch of the pulmonary artery. The anterior aspect of the oblique fissure is divided. The superior segmental pulmonary artery is also identified. The Cadiere Forceps is passed under the superior segmental pulmonary artery, a vessel loop is passed underneath and used to encircle and elevate the vessel, and the vessel is divided with a stapler with a white vascular cartridge introduced from a medial to lateral direction. Next the lower lobe artery is encircled and divided in a similar fashion with a white cartridge.
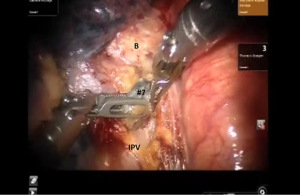
The lung is elevated and retracted medially. The Cadiere Forceps is passed from a medial to lateral direction under the IPV, a vessel loop is used to encircle and elevate the vein. The IPV is divided using a stapler with a white vascular load introduced from inferior to superior direction.
Preparation of the left mainstem bronchus (LB) and left upper lobe bronchus (UL) is similar to what has been outlined for sleeve right lower lobectomy (Figures 22,23). The anastomosis is started on the superior aspect of the cartilaginous portion of the LB which is at 12 o’clock position and the superior aspect of the cartilaginous portion of the UL which is 2 o’clock position and carried in an anti-clockwise fashion reapproximating the cartilaginous bronchus of the LB and UL ending at the inferior junction between the cartilaginous and membranous walls. 3-0 STRATAFIX (S1) is passed outside in at 12 o’clock position on LB and inside out at 1–2 o’clock position on UL (Figures 24,25). The suture is then tightened by passing it through the “eyelet”. For the second bite, the S1 needle is passed from inside out at the 3 to 4 o’clock position on UL and outside in at the 11 o’clock position on LB. For the third bite, the S1 needle is passed inside out at 5–6 o’clock position on UL and outside in at the 10 o’clock position on LB. For the fourth bite, the S1 needle is passed inside out at the 6–7 o’clock position on UL and outside in from the 9 o’clock position on LB. The suturing is continued in an anti-clockwise manner until the inferior border between the cartilaginous and membranous wall of the bronchi (Figures 26-29). A second 3-0 STRATAFIX suture (S2) is started at the superior border of the membranous bronchus and used to close the membranous bronchus (Figure 30). S1 and S2 are tied in the lower border of the membranous bronchus placing the knot onto the cartilaginous portion of the bronchi. The integrity of the anastomosis is checked as has been outlined previously (Figure 31).
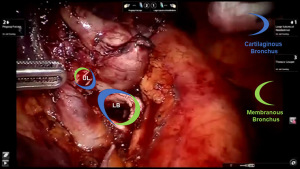
Comment
Sleeve lobectomy is an oncologically valid alternative to pneumonectomy and leads to better postoperative pulmonary function without any decrease in long-term survival.
Sleeve lobectomy has been shown to confer a benefit in early overall and disease-free survival in matched cohorts, when compared to pneumonectomy (20,21).
By virtue of superior visualization and more precise instrument maneuverability the robotic platform facilitates sleeve lobectomy using minimally invasive techniques. When compared with VATS and open surgery, robotic sleeve lobectomy has been shown to be a safe procedure with similar oncologic survival for centrally located non-small cell lung tumors (7).
The key to excellent and consistent results with robotic sleeve lobectomy are: (I) patient selection; (II) extensive experience with robotic lobectomy and segmentectomy procedures; and (III) a methodical approach to the bronchial anastomosis as has been described in this paper.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Richard Lazzaro) for the series “Robotic Anatomic Pulmonary Resection” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-157/coif). The series “Robotic Anatomic Pulmonary Resection” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this article and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Allison PR. Course of thoracic surgery in Groningen. Ann R Coll Surg 1954;25:20-2.
- Khargi K, Duurkens VA, Verzijlbergen FF, et al. Pulmonary function after sleeve lobectomy. Ann Thorac Surg 1994;57:1302-4. [Crossref] [PubMed]
- Angeletti CA, Janni A, Macchiarini P, et al. Functional results of bronchial sleeve lobectomy. Eur J Cardiothorac Surg 1991;5:410-3. [Crossref] [PubMed]
- Terzi A, Lonardoni A, Falezza G, et al. Sleeve lobectomy for non-small cell lung cancer and carcinoids: results in 160 cases. Eur J Cardiothorac Surg 2002;21:888-93. [Crossref] [PubMed]
- Lausberg HF, Graeter TP, Wendler O, et al. Bronchial and bronchovascular sleeve resection for treatment of central lung tumors. Ann Thorac Surg 2000;70:367-71; discussion 371-2. [Crossref] [PubMed]
- Zhou S, Pei G, Han Y, et al. Sleeve lobectomy by video-assisted thoracic surgery versus thoracotomy for non-small cell lung cancer. J Cardiothorac Surg 2015;10:116. [Crossref] [PubMed]
- Schmid T, Augustin F, Kainz G, et al. Hybrid video-assisted thoracic surgery-robotic minimally invasive right upper lobe sleeve lobectomy. Ann Thorac Surg 2011;91:1961-5. [Crossref] [PubMed]
- Wei S, Chen M, Chen N, et al. Feasibility and safety of robot-assisted thoracic surgery for lung lobectomy in patients with non-small cell lung cancer: a systematic review and meta-analysis. World J Surg Oncol 2017;15:98. [Crossref] [PubMed]
- O'Sullivan KE, Kreaden US, Hebert AE, et al. A systematic review and meta-analysis of robotic versus open and video-assisted thoracoscopic surgery approaches for lobectomy. Interact Cardiovasc Thorac Surg 2019;28:526-34. [Crossref] [PubMed]
- Merritt RE, D'Souza DM, Abdel-Rasoul M, et al. Analysis of trends in perioperative outcomes in over 1000 robotic-assisted anatomic lung resections. J Robot Surg 2023;17:435-45. [Crossref] [PubMed]
- Ishikawa N, Sun YS, Nifong LW, et al. Thoracoscopic robot-assisted bronchoplasty. Surg Endosc 2006;20:1782-3. [Crossref] [PubMed]
- Qiu T, Zhao Y, Xuan Y, et al. Robotic sleeve lobectomy for centrally located non-small cell lung cancer: A propensity score-weighted comparison with thoracoscopic and open surgery. J Thorac Cardiovasc Surg 2020;160:838-846.e2. [Crossref] [PubMed]
- Cerfolio RJ. Robotic sleeve lobectomy: technical details and early results. J Thorac Dis 2016;8:S223-6. [PubMed]
- Jo MS, Kim DY, Jeong JY, et al. Robotic sleeve lobectomy with four arms for lung cancer centrally located in the right lower lobe: a case report. J Cardiothorac Surg 2017;12:108. [Crossref] [PubMed]
- Durand M. Robotic bronchial sleeve resections: technical details and early results. Mini-invasive Surg 2019;3:35. [Crossref]
- Cerfolio RJ, Bryant AS, Skylizard L, et al. Initial consecutive experience of completely portal robotic pulmonary resection with 4 arms. J Thorac Cardiovasc Surg 2011;142:740-6. [Crossref] [PubMed]
- Cerfolio RJ. Total port approach for robotic lobectomy. Thorac Surg Clin 2014;24:151-6. v. [Crossref] [PubMed]
- Gharagozloo F, Meyer M, Tempesta B. Robotic Lobectomy: Experience with 638 Consecutive Cases. Surg Technol Int 2020;36:251-6. [PubMed]
- Gharagozloo F, Meyer M, Tempesta B, et al. Robotic Lobectomy. Chapter in: Gharagozloo F, Patel V, Giulianotti P, et al. editors. Robotic Surgery. Second Edition, Springer; 2021.
- Pagès PB, Mordant P, Renaud S, et al. Sleeve lobectomy may provide better outcomes than pneumonectomy for non-small cell lung cancer. A decade in a nationwide study. J Thorac Cardiovasc Surg 2017;153:184-95.e3. [Crossref] [PubMed]
- Li C, Zhou B, Han Y, et al. Robotic sleeve resection for pulmonary disease. World J Surg Oncol 2018;16:74. [Crossref] [PubMed]
Cite this article as: Gharagozloo F. Robotic sleeve lower lobectomy. Curr Chall Thorac Surg 2023;5:45.