
Surgical risk and survival impact of octogenarian lung cancer patients compared to those of younger patients undergoing surgery
Highlight box
Key findings:
• Oncological survival of octogenarian patients with lung cancer undergoing surgery was almost equivalent to that of younger patients with acceptable morbidity and mortality.
What is known and what is new?
• It is known that the outcomes of surgery for octogenarian patients with lung cancer were almost acceptable and reasonable.
• Our findings are reasonable even when compared with those of younger patients.
What is the implication, and what should change now?
• Lower body mass index was a notable poor prognostic factor, especially for octogenarian patients. Therefore, perioperative nutritional intervention and rehabilitation to maintain muscle weight against sarcopenia might improve their survival.
Introduction
Background
The proportion of older adults has been increasing globally in both developing and developed countries (1). Consequently, the number of older patients with lung cancer undergoing surgery also increased (2-4). According to previous studies, the postoperative morbidity and mortality rates of octogenarian patients undergoing lung cancer surgery were quite acceptable and the overall survival rate was reasonable (5-10). However, the patients were retrospectively analyzed, without comparison with the outcomes of younger individuals. Additionally, survival impact after pulmonary resection in older patients was unclear because there was a considerable rate of non-cancer-related deaths associated with cardiovascular and cerebrovascular events, or pneumonia, which negatively impacted overall survival.
Rationale and knowledge gap
Several clinical characteristics, such as male sex, pathological stage, Glasgow prognostic score, Charlson comorbidity index (CCI), prognostic nutritional index, and pathological stage, were reported as significant risk factors for long-term survival after lung cancer surgery in older patients (5,7,9-11). However, the difference in survival after lung cancer surgery between older and younger patients has not been clarified. Especially, oncological survival of older patients undergoing surgery compared with that of the younger population has not been elucidated to date.
Objective
We aimed to evaluate whether the perioperative surgical results and postoperative survival rates in octogenarian patients with lung cancer compared to those of younger patients were superior or inferior. We also aimed to analyze whether a difference exists in prognostic risk factors between the older and younger population and to clarify whether there was survival vulnerability so as to improve surgical outcomes, particularly in older patients. We present the following article in accordance with the STROBE reporting checklist (available at https://ccts.amegroups.com/article/view/10.21037/ccts-22-8/rc).
Methods
We performed this retrospective cohort study using a clinical database at a single institution. A total of 1,511 consecutive patients with primary lung cancer who underwent surgery at our institution from 2006 to 2019 were investigated. We included cases of complete resection for non-small cell lung cancer, excluding cases of small cell lung cancer (including combined small cell lung cancer), palliative surgery, history of multiple lung cancer, carcinoma in situ, patients with missing data (especially preoperative clinical characteristics), and patients who underwent preoperative treatment. Among them, we included 1,260 patients with lung cancer. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Research Review Board at Kansai Medical University, Osaka approved this study on September 8, 2021 (approval No. 2021135) and individual consent for this retrospective analysis was waived.
We gathered the clinical database from the Kansai Medical University Hospital, in which the patients underwent surgery, and conducted data collection from September 9, 2021 to December 31, 2021. All relevant data are presented in this paper and its Supporting Information files. Clinical data obtained included age at operation, sex, percentage of vital capacity (%VC), forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), body mass index (BMI), smoking history, preoperative carcinoembryonic antigen (CEA) level, standard uptake value max (SUVmax) of tumor, CCI, operative procedure, histology, pathological stage, postoperative complication(s), postoperative hospital mortality, hospital stay, postoperative adjuvant therapy, and observation time (12). Postoperative complications were defined by chart review, according to the Clavien-Dindo classification (13). Adverse events graded II and over II were defined as postoperative complications, whereas those graded III and over III were defined as severe complications. To investigate the result of lung cancer surgery in elderly patients, we assigned the patients to three groups, according to age, as follows: young (<60 years; Y group), middle-aged (60–79 years; M group), and old (≥80 years; O group). We defined the cutoff value of age for older patient as ≥80 years, partly because there were many publications for studying older patients with lung cancer undergoing surgery at an age ≥80 years; the results were almost similar, indicating that the surgical outcomes were acceptable and reasonable. Although elderly individuals are considered those aged >70 or 75 years, this definition might not be suitable for lung cancer surgery since the mean or median age of patients undergoing surgery is approximately 70 years and almost half the cohort was included in the O group. An age of ≤60 years was considered as a cut-off value for the younger individuals whose clinical characteristics were completely different from those aged ≥80 years. Therefore, we can clarify the true surgical results of older patients, and compared them with the corresponding of younger patients and patients with lung cancer aged 60–79 years undergoing surgery in terms of postoperative complications and long-term outcomes. The general indications for surgery, especially for octogenarians, were as follows: performance status, 0–1; postoperative predicted forced expiratory volume in 1 s >800 mL (14); clinical stage, I–IIIA expected to be completely resectable; and mental status, not senile. The overall survival time was calculated as the time from the date of surgery to the date of death or the last follow-up. The cancer-specific survival time was calculated from the date of surgery to that of death due to lung cancer. Recurrence-free survival time was calculated from the date of surgery to the date of recurrence. The tumor stage was determined according to the eighth edition of the International Union Against Cancer (15). The tumor histological type was determined according to the third edition of the World Health Organization Classification of Tumors (16).
Statistical analyses
We analyzed the clinical characteristics of the three groups using the Kruskal-Wallis test and the clinical characteristics of the two groups using the Mann-Whitney test with Bonferroni correction. We calculated survival time using the Kaplan-Meier method and assessed the differences with the log-rank test. The cutoff values for BMI to draw the survival curves were set at the bottom quartile; 20.4 kg/m2 for the entire cohort and 22.6 kg/m2 for the O group. We performed univariate and multivariate analyses for overall survival using the Cox proportional hazards model. Additionally, we analyzed risk factors for postoperative complication grade ≥ II using logistic regression analysis among the Y, M, and O groups. Univariate analysis was performed for each variable first; then, variables with P<0.2 were selected for multivariate analysis. We performed a Kruskal-Wallis test, Mann-Whitney test with Bonferroni correction, Kaplan-Meier method with log-rank test, and the Cox proportional hazards regression and logistic regression analysis using JMP software version 12 (SAS Institute Inc., Cary, NC, USA). Statistical significance was set at P<0.05.
Results
A total of 1,511 consecutive patients with primary lung cancer who underwent surgery at our institution from January 2006 to December 2019 were investigated. Cases of small cell lung cancer (including combined small cell lung cancer) (n=16), palliative surgery (n=93), history of multiple lung cancer (n=51), carcinoma in situ (n=24), patients with missing data or inappropriate case (n=27), and patients who underwent preoperative treatment (n=40) were excluded. The patient selection flow chart is presented in Figure S1. Among the series of lung cancer surgeries, we included 1,260 patients with lung cancer, and the number of patients in the three groups were as follows: Y group, n=170; M group, n=958; and O group, n=132. In the total cohort, the 30- and 90-day postoperative mortality rates were 0.2% (n=3) and 0.3% (n=4), respectively. The preoperative clinical characteristics and postoperative outcomes of the Y, M, and O groups are shown in Table 1. The patients in the O group had significantly lower %VC, lower FEV1/FVC and BMI, higher CCI, higher percentage of wedge resection or segmentectomy, non-adenocarcinoma histology, postoperative complications, lower proportion of adjuvant therapy, longer postoperative hospital stay, and shorter postoperative observation time (P<0.05 for all). However, there were no significant differences in sex, CEA level, SUVmax, pathological stage, and postoperative hospital mortality between the three groups (P>0.05 for all). The rates of all postoperative complications were significantly higher in the O group than in the Y group (P=0.01). However, the rates of severe complications and hospital mortality were not significantly different between the O and Y groups (P=0.26 and 0.33). The preoperative clinical characteristics and postoperative outcomes of the Y, M, and O groups limited to pathological stage I were almost the same as those of all stages (Table 2).
Table 1
| Clinical characteristics | Y group (n=170) | M group (n=958) | O group (n=132) | P value | |
|---|---|---|---|---|---|
| Age (years), mean [range] | 53.1 [33–59] | 70.6 [60–79] | 82.2 [80–89] | – | |
| Sex, n (%) | 0.10 | ||||
| Male | 112 (65.9) | 586 (61.2) | 71 (53.8) | ||
| Female | 58 (34.1) | 372 (38.8) | 61 (46.2) | ||
| Percentage of VC, mean (range) | 103.5 (60.3–153.8) | 99.3 (46.0–157.2) | 99.0 (54.7–143.5) | O/Y 0.03*; O/M 0.90; M/Y0.004* | |
| FEV1/FVC, mean (range) | 76.4 (51.8–97.8) | 72.7 (31.7–100.0) | 71.0 (30.6–96.3) | O/Y <0.001*; O/M 0.04*; M/Y <0.001* | |
| Pack-year index, n (%) | |||||
| ≤600 | 106 (62.4) | 484 (50.5) | 77 (58.3) | ||
| >600 | 53 (32.2) | 424 (44.3) | 52 (39.4) | ||
| Unknown | 11 (5.4) | 50 (5.2) | 3 (2.3) | 0.004* | |
| Body mass index, mean (range) | 23.3 (14.1–32.9) | 22.6 (14.2–38.4) | 22.1 (14.7–30.0) | O/Y 0.02; O/M 0.60; M/Y 0.048 | |
| CEA level, n (%) | |||||
| >5 | 38 (22.4) | 249 (26.0) | 33 (25.0) | ||
| ≤5 | 113 (66.5) | 573 (59.8) | 82 (62.1) | ||
| Unknown | 19 (11.1) | 136 (4.2) | 17 (12.9) | 0.43 | |
| Standard uptake valuemax, mean (range) | 5.53 (0.6–21.1) | 5.22 (0–23.9) | 6.15 (0–24.1) | O/Y 1.0; O/M 0.36; M/Y 0.30 | |
| Charlson comorbidity index, n (%) | |||||
| 0, 1 | 149 (87.6) | 693 (72.3) | 90 (68.2) | ||
| ≥2 | 21 (12.4) | 265 (27.7) | 42 (31.8) | <0.001* | |
| Procedure, n (%) | |||||
| Sublobar resection | |||||
| Wedge resection | 19 (11.2) | 135 (14.1) | 23 (17.4) | ||
| Segmentectomy | 4 (2.4) | 54 (5.6) | 9 (6.8) | ||
| Radical surgery | |||||
| Lobectomy | 146 (85.9) | 766 (80.0) | 100 (75.8) | ||
| Pneumonectomy | 1 (0.5) | 3 (0.3) | 0 (0.0) | 0.049* | |
| Histology, n (%) | |||||
| Adenocarcinoma | 135 (79.4) | 680 (71.1) | 87 (65.9) | ||
| Squamous cell carcinoma | 14 (8.2) | 198 (20.7) | 37 (28.0) | ||
| Others | 21 (12.4) | 79 (8.2) | 8 (6.1) | <0.001* | |
| Pathological stage, n (%) | |||||
| I | 119 (70.0) | 711 (74.2) | 96 (72.7) | ||
| II | 28 (16.5) | 139 (14.5) | 21 (15.9) | ||
| III | 23 (13.5) | 108 (11.3) | 15 (11.4) | 0.84 | |
| Postoperative complications grade II or over, n (%) | |||||
| Yes | 13 (7.6) | 155 (16.2) | 24 (18.2) | ||
| No | 157 (92.4) | 803 (83.8) | 108 (81.8) | 0.005* | |
| Postoperative complications grade III or over, n (%) | |||||
| Yes | 5 (2.9) | 58 (6.1) | 8 (6.1) | ||
| No | 165 (97.1) | 900 (93.9) | 124 (93.9) | 0.216 | |
| Postoperative hospital mortality, n (%) | |||||
| Yes | 0 (0.0) | 7 (0.7) | 0 (0.0) | ||
| No | 170 (100.0) | 958 (99.3) | 132 (100.0) | 0.33 | |
| Postoperative hospital stay (days), median [range] | 11 [5–51] | 11 [3–347] | 12 [4–125] | O/Y 0.02; O/M 0.09; M/Y 1.0 | |
| Postoperative adjuvant therapy, n (%) | |||||
| Yes | 70 (41.2) | 250 (26.1) | 6 (4.6) | ||
| No | 100 (58.8) | 708 (73.9) | 126 (95.5) | <0.001* | |
| Observation time (months), mean (range) | 52.3 (1.2–159.8) | 41.4 (0.23–76.0) | 22.6 (0.73–103.1) | O/Y <0.001*; O/M <0.001*; M/Y 0.002* | |
*, P value <0.05. Y group: patients aged <60 years; M group: patients aged 60–79 years; O group: patients aged ≥80 years. VC, vital capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; CEA, carcinoembryonic antigen.
Table 2
| Clinical characteristics | Y group (n=119) | M group (n=711) | O group (n=96) | P value | |
|---|---|---|---|---|---|
| Age (years), mean [range] | 53.1 [33–59] | 70.6 [60–79] | 82.2 [80–88] | – | |
| Sex, n (%) | 0.31 | ||||
| Male | 73 (61.3) | 426 (59.9) | 50 (52.1) | ||
| Female | 46 (38.7) | 285 (40.1) | 46 (47.9) | ||
| Percentage of VC, mean (range) | 103.4 (60.3–153.8) | 99.7 (46.0–157.2) | 98.1 (61.5–143.5) | O/Y 0.02*; O/M 0.30; M/Y 0.04* | |
| FEV1/FVC, mean (range) | 77.3 (51.8–97.8) | 72.8 (31.7–100) | 71.6 (45.5–96.3) | O/Y <0.001*; O/M 0.14; M/Y <0.001* | |
| Body mass index, mean (range) | 23.2 (14.1–32.9) | 22.6 (14.2–38.4) | 22.2 (14.7–30.0) | O/Y 1.0; O/M 1.0; M/Y 0.15 | |
| Pack-year index, n | |||||
| ≤600 | 74 | 380 | 57 | ||
| >600 | 38 | 288 | 36 | ||
| Unknown | 7 | 43 | 3 | 0.16 | |
| CEA level, n (%) | |||||
| >5 | 23 (19.3) | 161 (22.6) | 18 (18.8) | ||
| ≤5 | 84 (70.6) | 453 (63.7) | 65 (67.7) | ||
| Unknown | 12 (10.1) | 97 (13.7) | 13 (13.5) | 0.43 | |
| Standard uptake valuemax, mean (range) | 4.10 (0.6–13.6) | 4.14 (0–19.8) | 4.73 (0–16.2) | O/Y 1.0; O/M 1.0; M/Y1.0 | |
| Charlson comorbidity index, n (%) | |||||
| 0, 1 | 101 (84.9) | 506 (71.2) | 63 (65.6) | ||
| ≥2 | 18 (15.1) | 205 (28.8) | 33 (34.4) | 0.001* | |
| Procedure, n (%) | |||||
| Sublobar resection | |||||
| Wedge resection | 19 (16.0) | 125 (17.6) | 22 (22.9) | ||
| Segmentectomy | 4 (3.4) | 48 (6.8) | 6 (6.3) | ||
| Radical surgery | |||||
| Lobectomy | 96 (80.7) | 538 (75.7) | 68 (70.8) | ||
| Pneumonectomy | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0.24 | |
| Histology, n (%) | |||||
| Adenocarcinoma | 101 (84.9) | 535 (75.3) | 66 (68.8) | ||
| Squamous cell carcinoma | 7 (5.9) | 129 (18.1) | 27 (28.1) | ||
| Others | 11 (9.2) | 47 (6.6) | 3 (3.1) | <0.001* | |
| Postoperative complications grade II or over, n (%) | |||||
| Yes | 8 (6.7) | 104 (14.6) | 20 (20.8) | ||
| No | 111 (93.3) | 607 (85.4) | 76 (79.2) | 0.008* | |
| Postoperative complications grade III or over, n (%) | |||||
| Yes | 4 (3.4) | 42 (5.9) | 6 (6.3) | ||
| No | 115 (96.6) | 669 (94.1) | 90 (93.7) | 0.4752 | |
| Postoperative hospital mortality, n (%) | |||||
| Yes | 0 | 4 (0.6) | 0 | ||
| No | 119 (100.0) | 707 (99.4) | 96 (100.0) | 0.35 | |
| Postoperative hospital stay (days), median [range] | 10 [5–51] | 11 [3–347] | 11 [5–71] | O/Y 0.42; O/M 1.0; M/Y 1.0 | |
| Postoperative adjuvant therapy, n (%) | |||||
| Yes | 26 (21.9) | 103 (14.6) | 2 (2.1) | ||
| No | 93 (78.1) | 604 (85.4) | 94 (97.9) | <0.001* | |
| Observation time (months), mean (range) | 56.4 (4.2–154.7) | 44.2 (0.23–172.8) | 26.1 (0.73–103.1) | O/Y <0.001*; O/M <0.001*; M/Y 0.02* | |
*, P value <0.05. Y group: patients aged <60 years; M group: patients aged 60–79 years; O group: patients aged ≥80 years. VC, vital capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; CEA, carcinoembryonic antigen.
Kaplan-Meier survival curves for all 1,260 patients stratified by age group, including all stages of lung cancer, are shown in Figure 1A-1C. The 5-year overall survival rates for the Y, M, and O groups were 87.6% [95% confidence interval (CI), 80.5–92.3%], 76.0% (72.4–79.2%), and 66.4% (52.0–78.4%), respectively, indicating that the O group had significantly shorter overall survival (P<0.001). The 5-year cancer-specific and recurrence-free survivals of the three groups were 90.1% (83.6–94.2%) and 76.2% (68.4–82.5%), 83.8% (80.6–86.5%) and 69.8% (66.3–73.1%), and 77.8% (64.0–87.4%) and 69.2% (58.1–78.5%), respectively, which indicated that oncological survival in the O group was slightly lower or equivalent to that in the Y group (P=0.02 and 0.11).

In contrast to Kaplan-Meier survival curves that included all stages, those limited to pathological stage I of 926 patients stratified by age group are shown in Figure 2A-2C. The 5-year overall survival rates of Y, M, and O groups were 91.8% (83.7–96.1%), 81.9% (78.0–85.2%), and 71.6% (53.0–85.0%), respectively, which exhibited the same significant difference for all cohorts (P=0.04). In contrast, the 5-year cancer-specific and recurrence-free survivals of the three groups were 95.4% (88.3–98.3%) and 86.9% (78.7–92.3%), 90.5% (87.3–92.9%) and 82.2% (78.6–85.3%), and 85.1% (66.4–94.2%) and 80.4% (67.7–88.9%), respectively, indicating that the O group had almost equivalent oncological survival (P=0.27 and 0.14).

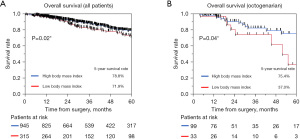
We analyzed the prognostic factors for overall survival among the 1,260 patients and among the Y, M, and O groups. The results are summarized in Tables 3,4. Univariate and multivariate analyses for overall survival revealed that increased age, lower %VC, CEA >5, higher SUVmax, histology of squamous cell carcinoma and other pathology, and higher pathological stage were independent negative prognostic factors in all cohorts (P<0.05) (Table 3). Negative independent prognostic factors stratified according to the three groups were as follows: CEA >5 and higher pathological stage in the Y group; lower %VC, histology of squamous cell carcinoma and other pathology, higher SUVmax, and pathological stage in M group; and lower BMI, higher SUVmax, and pathological stage in the O group (Table 4) (P<0.05 for all parameters). Kaplan-Meier survival curves among patients divided according to BMI are shown in Figure 3A and Figure 3B. The 5-year overall survival rates of patients with higher and lower BMI values were 78.8% (95% CI, 75.4–81.9%) and 71.9% (95% CI, 65.0–77.8%) for all patients and 75.4% (95% CI, 60.6–86.0%) and 37.0% (95% CI, 13.3–69.2%) for the O group, with statistical significance (P=0.02 and 0.04, respectively), indicating that lower BMI values had a remarkable impact on the O group. Moreover, we analyzed risk factors for postoperative complication grade II and over II by univariate and, multivariate analysis and the results were as followed: higher age and histology of squamous cell carcinoma for the Y group, male sex, and lower vital capacity and lower FEV1/FVC for M group, and lower CCI for the O group, respectively (P<0.05).
Table 3
| Variables | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | ||
| Age (years) | 1.03 (1.02–1.05) | <0.0001 | 1.04 (1.01–1.06) | 0.007* | |
| Sex (male/female) | 2.23 (1.66–3.04) | <0.0001 | 1.28 (0.78–2.13) | 0.33 | |
| Percentage of VC | 0.98 (0.97–0.98) | <0.0001 | 0.98 (0.97–0.99) | 0.002* | |
| FEV1/FVC | 0.99 (0.97–1.00) | 0.08 | 1.01 (0.98–1.02) | 0.83 | |
| Body mass index | 0.97 (0.93–1.01) | 0.16 | 0.95 (0.90–0.10) | 0.10 | |
| Pack years (>600/≤600) | 2.09 (1.60–2.74) | <0.001 | 1.13 (0.72–1.83) | 0.58 | |
| CEA (>5/≤5) | 2.08 (1.58–2.73) | <0.001 | 1.73 (1.19–2.50) | 0.004* | |
| Charlson comorbidity index (≥2/0 or 1) | 1.58 (1.20–2.07) | 0.001 | 1.32 (0.88–1.96) | 0.18 | |
| Sublobar resection (wedge resection or segmentectomy)/radical surgery (lobectomy or pneumonectomy) | 1.01 (0.73–1.37) | 0.95 | |||
| Standard uptake valuemax | 1.15(1.12–1.18) | <0.001 | 1.12 (1.08–1.17) | <0.001* | |
| Histology/adenocarcinoma | 1 | 1 | |||
| Squamous cell carcinoma | 2.21 (1.64–2.94) | <0.001 | 1.00 (0.62–1.58) | 0.99 | |
| Others | 2.27 (1.53–3.27) | <0.001 | 1.81 (1.01–3.10) | 0.045* | |
| Postoperative complication (yes/no) | 2.04 (1.50–2.74) | <0.001 | 1.23 (0.79–1.88) | 0.35 | |
| Postoperative adjuvant therapy (yes/no) | 1.21 (0.93–1.58) | 0.15 | 1.11 (0.73–1.67) | 0.61 | |
| Pathology stage I | 1 | 1 | |||
| II | 2.21 (1.60–3.03) | <0.001 | 1.60 (0.98–2.56) | 0.06 | |
| III | 3.74 (2.73–5.08) | <0.001 | 2.90 (1.80–4.60) | <0.001* | |
*, P value <0.05. VC, vital capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; CEA, carcinoembryonic antigen; 95% CI, 95% confidence interval.
Table 4
| Variables | Multivariate analysis | |||||||
|---|---|---|---|---|---|---|---|---|
| Y group | M group | O group | ||||||
| Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | Hazard ratio (95% CI) | P value | |||
| Age (years) | 1.02 (0.98–1.07) | 0.29 | ||||||
| Sex (male/female) | 4.90 (0.08–858.7) | 0.47 | 1.37 (0.79–2.39) | 0.27 | 3.71 (0.91–22.79) | 0.07 | ||
| Percentage of VC | 0.98 (0.96–0.99) | <0.001* | ||||||
| FEV1/FVC | 1.01 (0.88–1.15) | 0.92 | ||||||
| Body mass index | 0.77 (0.61–0.95) | 0.01* | ||||||
| Pack-year index (>600/≤600) | 19.31 (0.93–1004.4) | 0.06 | 1.02 (0.62–1.73) | 0.93 | ||||
| CEA (>5/≤5) | 9.68 (1.40–119.1) | 0.02* | 1.40 (0.93–2.08) | 0.11 | ||||
| Charlson comorbidity index (≥2/0 or 1) | 1.41 (0.91–2.17) | 0.12 | ||||||
| Sublobar resection (wedge resection or segmentectomy)/radical surgery (lobectomy or pneumonectomy) | 11.48 (0.93–236.0) | 0.06 | ||||||
| Histology/adenocarcinoma | 1 | 1 | ||||||
| Squamous cell carcinoma | 0.10 (0.001–5.01) | 0.25 | 0.89 (0.53–1.49) | 0.66 | ||||
| Others | 0.79 (0.02–18.07) | 0.88 | 2.12 (1.15–3.74) | 0.02* | ||||
| Standard uptake valuemax | 1.48 (0.98–2.22) | 0.04* | 1.10 (1.05–1.16) | <0.001* | 1.18 (1.03–1.36) | 0.01* | ||
| Postoperative complication (yes/no) | 1.13 (0.70–1.77) | 0.61 | ||||||
| Postoperative adjuvant chemotherapy (yes/no) | 0.64 (0.38–1.06) | 0.09 | ||||||
| Pathological stage I | 1 | 1 | 1 | |||||
| II | 2.97e–9 (0–17.67) | 0.45 | 1.94 (1.11–3.32) | 0.02* | 2.64 (0.42–16.05) | 0.29 | ||
| III | 23.48 (2.22–456.3) | 0.01* | 3.34 (1.87–5.92) | <0.001* | 8.90 (1.76–46.09) | 0.009* | ||
*, P value <0.05. VC, vital capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; CEA, carcinoembryonic antigen; 95% CI, 95% confidence interval.
Discussion
Key findings
The number of older adults in Japan has been rapidly increasing and a similar trend is observed globally (17). According to annual reports from the Japanese Association of Thoracic Surgery (3,4), the number of older patients (≥80 years) with lung cancer who underwent surgery in 2017 increased 1.5-fold to 13.1% (5,779/44,140), compared to 8.7% (2,273/26,092), which was recorded 10 years previously. Several studies have compared the surgical results of octogenarian patients with lung cancer to those of septuagenarians, which was likely to almost same as two groups (18-20). In contrast, little is known regarding the difference in surgical results between octogenarians and younger patients, thus far. Therefore, in the present study, we demonstrated the surgical outcomes of octogenarian lung cancer patients compared with those of younger patients in detailed investigations. Our results revealed that the overall survival of the O group was lower than that of the younger patients, due to short life expectancy; however, their oncological survival, particularly for pathological stage I cases, appeared to be equivalent to those of the younger patients, with no increased surgical mortality, which was not clearly shown in past publications.
Explanations of findings
In the background, the higher prevalence of wedge resection or segmentectomy in the O group was considered to contribute to the reduction of severe complications and the increase in survival time (Figures 2,3). Less invasive procedures in the O group may also have contributed to the reduction in postoperative mortality, which was as low as that of younger patients. A recent Japanese nationwide study suggested that wedge resection was an acceptable oncological procedure for selected comorbid octogenarian patients with early-stage lung cancer, which supports our results (21). Based on this evidence, surgical treatment for octogenarians was justified and acceptable, while being cautious for perioperative higher morbidity. Furthermore, the proportion of adjuvant therapy was significantly lower in the O group. Among the participants in the O group, we did not generally perform adjuvant therapy, due to age-related organ damage and intolerance of physical and mental conditions for chemotherapy based on a daily clinical conference conducted on a case-by-case basis. As a result, the lower proportion of adjuvant therapy might be one reason for the poor survival of the O group. As for the appreciably lower observation time of O group (mean: 22.6 months) than that of the other two groups (Y group: 52.3 months; M group: 41.4 months), several reasons can be assumed, partly because more recent patients had undergone surgery and partly because increased non-cancer related deaths. Additionally, the life-expectancy period was very short in our cohort. Regardless of the short observation time, we considered these to be reasonable results and that a future study with a longer observation time will also ultimately confirm the trend we observed.
Comparison with similar researches
As an alternative procedure, stereotactic ablative radiation therapy (SBRT) is generally indicated for high-risk older patients with impaired cardiopulmonary function, lower performance status, or senile mental condition, based on the surgeon’s decision related to case-by-case peculiarities. It was reported that SBRT resulted in low postoperative morbidity but worse long-term survival due to both oncological and non-oncological events compared with patients undergoing surgery (22). Therefore, we should carefully consider the indication and surgical procedure in octogenarian patients with lung cancer who have comorbidities that are being controlled. Although challenging, it is very important to select the optimal indication for older patients to ensure a reasonable outcome.
Concerning short-term outcomes, postoperative morbidity and mortality of octogenarian lung cancer patients were reported to be 29.9–55.8% and 1.0–4.2%, respectively, suggesting that it appeared worse but was generally acceptable (23). According to another study, the cumulative rate of complication for patients ≥70 years was not significantly lower than that of patients aged <70 years (40.0% vs. 36.3%, P=0.07). However, the 30-day mortality was significantly higher (3.6% vs. 2.2%, P=0.01) (24). Similar results were also reported among octogenarian patients from a database analysis in the Netherlands (25). Conversely, in a limited case-control study that compared surgical results between octogenarian and septuagenarian patients with lung cancer, there was no significant difference in surgical morbidity and mortality rates using propensity score matching (18,19). In the present study, the total rate of postoperative complications of octogenarian patients undergoing lung cancer surgery significantly increased in chronological order; however, severe complications and mortality did not increase in octogenarian patients compared to younger patients (Table 1). It is believed that these results reflect that the surgeon appropriately selected operable patients and chose the optimal procedure based on the patient’s comorbidity, cardiopulmonary function, and performance status. Although the rate of postoperative complications and mortality in octogenarian patients varied in previous reports and appeared to be relatively higher than those of younger patients, this was dependent on the patient’s characteristics, type of procedures, and institute. In addition, we performed a multivariate analysis to detect risk factors for postoperative complications among each group and demonstrated that those we identified were consistent with prior reports and different in groups stratified by age (Table 5) (8,10). However, the significant risk index in patients of the O group revealed lower CCI according to our result. It was considered that patients with higher CCIs were likely to be indicated for the safer procedure of wedge resection. In contrast, patients with lower CCIs may be more likely to undergo radical surgery of lobectomy. As a result, the latter tended to have more complications than patients with higher CCIs in this cohort. Especially, a sublobar resection procedure might reduce postoperative complications for high-risk patients with higher CCIs, and a radical lobectomy procedure for patients with lower CCIs might have a higher likelihood of postoperative complications, which should be carefully monitored, postoperatively. Further, the data demonstrated “real world” outcomes of lung cancer surgery for octogenarian patients, which indicated that such patients can undergo radical surgery via lobectomy or wedge resection as appropriate providing that they meet the criteria for surgery.
Table 5
| Variables | Y group | M group | O group | |||||
|---|---|---|---|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |||
| Age (years) | 1.45 (1.06–2.41) | 0.01* | ||||||
| Sex (male/female) | 1.55 (0.17–33.93) | 0.71 | 1.95 (1.08–3.59) | 0.03* | ||||
| Percentage of VC | 0.96 (0.91–1.01) | 0.15 | 0.97 (0.95–0.98) | <0.001* | ||||
| FEV1/FVC | 0.97 (0.95–0.99) | 0.03* | ||||||
| Body mass index | 0.95 (0.88–1.02) | 0.13 | ||||||
| Pack years (>600/≤600) | 0.84 (0.48–1.45) | 0.52 | ||||||
| CEA (>5/≤5) | 1.32 (0.82–2.10) | 0.25 | 2.58 (0.88–7.57) | 0.08 | ||||
| Charlson comorbidity index (≥2/0 or 1) | 0.88 (0.52–1.47) | 0.63 | 0.25 (0.04–0.09) | 0.048* | ||||
| Radical surgery (lobectomy or pneumonectomy)/sublobar resection (wedge resection or segmentectomy) | 2.22 (0.95–5.93) | 0.07 | 1.66 (0.45–8.12) | 0.47 | ||||
| Histology/adenocarcinoma | 1 | 1 | 1 | |||||
| Squamous cell carcinoma | 10.06 (1.45–86.28) | 0.02* | 1.13 (0.61–2.07) | 0.69 | 2.07 (0.66–6.34) | 0.21 | ||
| Others | 3.20e–9 (0–2.85) | 0.18 | 1.32 (0.60–2.77) | 0.47 | 1.73e–7 (0–1.76) | 0.12 | ||
| Standard uptake valuemax | 1.11 (0.89–1.38) | 0.33 | 1.05 (0.98–1.12) | 0.16 | ||||
| Pathological stage I | 1 | |||||||
| II | 1.12 (0.59–2.07) | 0.71 | ||||||
| III | 1.07 (0.54–2.07) | 0.84 | ||||||
*, P value <0.05. VC, vital capacity; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; CEA, carcinoembryonic antigen; 95% CI, 95% confidence interval.
Regarding survival for octogenarian lung cancer surgery, the 5-year overall survival rate was reported as 49.9–72.8%, which was considered acceptable, regardless of the disadvantages of age, comorbidities, and less opportunity for adjuvant therapy (8,10,19). As mentioned in other studies, the overall, recurrence-free, and cancer-specific survival rates of octogenarian patients with lung cancer were equivalent to those of septuagenarians, using propensity score matching (18,19). In this study, we demonstrated the oncological impact of surgery for octogenarian patients, showing that the 5-year cancer-specific and recurrence-free survival of the O group at pathological stage I was not significantly lower than that of the younger populations, using raw data and not adjusting for clinical characteristics (Figure 2B,2C). Considering the patients’ background, the octogenarian patients had a considerable rate of non-cancer death prior to cancer recurrence, which may have obscured and decreased the chance of cancer recurrence or death in the early postoperative period. Our past study supports the evidence that the number of non-cancer-related deaths exceeded that of cancer deaths from surgery until approximately 1.5 years. However, subsequently, the number of cancer deaths was a cross over from non-cancer death, suggesting that non-cancer death had a considerable impact on survival in the earlier postoperative period among octogenarians (26). Based on these trends, the oncological survival outcome of octogenarians was considered as a result, which was consequently balanced and adjusted for non-cancer and cancer events. Therefore, we should carefully perform radical surgery for eligible octogenarian patients with lung cancer, with considerations of comorbidity to ensure that oncological survival is guaranteed.
Regarding prognostic factors in octogenarian patients undergoing lung cancer surgery, sex, histology, respiratory function, Glasgow prognostic factor, CCI, extent of lung resection, pathological stage, and BMI have been reported as significant risk factors (5-10). Our results were quite consistent with the results of prior reports including all cohorts; however, the percentage of VC, CEA, and histology were not consistent in the group of octogenarians. This was considered to be attributed to impaired cardiopulmonary function and considerable non-cancer related death in the elderly cohort, which affected the difference in prognostic indices, compared to younger patients. In contrast, selected lower BMI was strongly associated with worse survival in all cohorts and in the octogenarian cohort, demonstrating that higher BMI, as well as oncological variables, such as lower value of SUVmax and lower pathological staging, had a potentially favorable index (27). In this study, we attempted to assess sarcopenia by using the BMI, a simple variable representing preoperative weight loss and the potential influence of cachexia on surgical results among patients stratified by age. Our results confirmed that lower BMI was a strong factor for long-term survival among octogenarians, demonstrating that nutritional condition is of great value for patients with lung cancer preoperatively, especially for older patients, in addition to the CCI. Therefore, even without collecting detailed, complicated variables, such as muscle volume and albumin value, we can detect high-risk patients by assessing lower BMI preoperatively. Originally, a higher BMI, is a well-known negative risk factor for mortality in cardiovascular diseases and type II diabetes as well as in various malignancies (28). In contrast, obesity appears to have a protective effect on survival in lung cancer patients, which is the so-called “obesity paradox” (29). According to a previous study, a “metabolically healthy obese” patient with lung cancer who possesses considerable muscle volume tends to have a good prognosis (30). Particularly, in Asian populations, higher BMI values in overweight patients (25–30 kg/m2) and mildly obese individuals may provide sufficient nutritional reserves for adequate response to stressors, which is a possible explanation for the impact of the obesity paradox in all cohorts as well as in octogenarian patients with lung cancer. Conversely, lower BMI values are closely associated with “sarcopenia,” which is an age-related reduction in body weight with muscle shrinkage (31). This was also a strong negative predictor in patients with lung cancer undergoing surgery (32). Recent publications mentioned a significant correlation between sarcopenia and lower oncological survival (cancer-specific and recurrence-free survival) among patients undergoing lung cancer surgery, which supports our results (33,34). Therefore, lower BMI, reflecting sarcopenia, was notable among octogenarians; an interventional approach, including perioperative positive nutritional support and physical training, as a method of rehabilitation to maintain body weight and muscle volume, may improve survival, particularly for octogenarian patients (35). A prospective study to assess individual perioperative management could confirm the reason for the prolonged survival.
Strengths and limitations
This study had some limitations. First, selection bias cannot be ruled out, because older patients with multiple comorbidities may or may not have been chosen for surgery at the attendant surgeons’ discretion and the selected surgical procedure also varied depending on the patient’s condition. Second, the study was retrospectively conducted at a single institution. Third, the observation period was >10 years, during which time chemotherapeutic agents changed; this could have affected the survival rate. Fourth, we could obtain only limited clinical characteristics that affect surgical results, and assessment of patients’ pre- and postoperative status, mental and cognitive condition, and performance status might be desirable to analyze postoperative course more precisely, especially for older patients. Nevertheless, we demonstrated the oncological feasibility of lung cancer surgery in elderly patients versus younger patients, with acceptable surgical complications. Moreover, lower BMI was one valuable prognostic factor, especially in the older cohort, which is a possible, simple candidate for intervention in the course of lung cancer surgery. We believe this information would enable surgeons to suitably perform radical surgery for elderly patients.
Implications and actions needed
Lower BMI, reflecting sarcopenia and lower nutritional status, was notable in the O group, and perioperative nutritional support and rehabilitation to maintain body weight with muscle volume might improve their overall survival. A large prospective study promoting nutritional intervention and physical activity in daily exercise for elderly patients with lung cancer might contribute to survival in this population.
Conclusions
We showed that the oncological outcomes of cancer-specific and recurrence-free survival among octogenarian patients with lung cancer undergoing surgery were slightly lower or almost equivalent to those of younger populations, regardless of differences in clinical characteristics. Prognostic factors for lung cancer patient were varied among age groups, especially for older patients, lower BMI was notable; perioperative nutritional intervention might contribute to prolonging their postoperative survival.
Acknowledgments
This manuscript was presented as an e-poster at the 29th Meeting of the European Society of Thoracic Surgeons Virtual Meeting (June 20-22, 2021). We are very grateful to Editage for English proofreading.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://ccts.amegroups.com/article/view/10.21037/ccts-22-8/rc
Data Sharing Statement: Available at https://ccts.amegroups.com/article/view/10.21037/ccts-22-8/dss
Peer Review File: Available at https://ccts.amegroups.com/article/view/10.21037/ccts-22-8/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-22-8/coif). TM serves as an unpaid editorial board member of Current Challenges in Thoracic Surgery from November 2021 to October 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The Research Review Board at Kansai Medical University, Osaka approved this study on September 8, 2021 (approval No. 2021135) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. (2015). World report on ageing and health. World Health Organization. Available online: https://apps.who.int/iris/handle/10665/186463 (2021/12/27 access)
- Owonikoko TK, Ragin CC, Belani CP, et al. Lung cancer in elderly patients: an analysis of the surveillance, epidemiology, and end results database. J Clin Oncol 2007;25:5570-7. [Crossref] [PubMed]
- Committee for Scientific Affairs. Thoracic and cardiovascular surgery in Japan during 2007. Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2009;57:488-513. [Crossref] [PubMed]
- Committee for Scientific Affairs. Thoracic and cardiovascular surgeries in Japan during 2017: Annual report by the Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg 2020;68:414-49. [Crossref] [PubMed]
- Birim O, Maat AP, Kappetein AP, et al. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg 2003;23:30-4. [Crossref] [PubMed]
- Dominguez-Ventura A, Cassivi SD, Allen MS, et al. Lung cancer in octogenarians: factors affecting long-term survival following resection. Eur J Cardiothorac Surg 2007;32:370-4. [Crossref] [PubMed]
- Fanucchi O, Ambrogi MC, Dini P, et al. Surgical treatment of non-small cell lung cancer in octogenarians. Interact Cardiovasc Thorac Surg 2011;12:749-53. [Crossref] [PubMed]
- Matsuoka K, Yamada T, Matsuoka T, et al. Significance of Body Mass Index for Postoperative Outcomes after Lung Cancer Surgery in Elderly Patients. World J Surg 2018;42:153-60. [Crossref] [PubMed]
- Hino H, Murakawa T, Ichinose J, et al. Results of Lung Cancer Surgery for Octogenarians. Ann Thorac Cardiovasc Surg 2015;21:209-16. [Crossref] [PubMed]
- Hino H, Karasaki T, Yoshida Y, et al. Risk factors for postoperative complications and long-term survival in lung cancer patients older than 80 years. Eur J Cardiothorac Surg 2018;53:980-6. [Crossref] [PubMed]
- Watanabe I, Kanauchi N, Watanabe H. Preoperative prognostic nutritional index as a predictor of outcomes in elderly patients after surgery for lung cancer. Jpn J Clin Oncol 2018;48:382-7. [Crossref] [PubMed]
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987;40:373-83. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Sawabata N, Miyaoka E, Asamura H, et al. Japanese lung cancer registry study of 11,663 surgical cases in 2004: demographic and prognosis changes over decade. J Thorac Oncol 2011;6:1229-35. [Crossref] [PubMed]
- Rami-Porta R, Bolejack V, Giroux DJ, et al. The IASLC lung cancer staging project: the new database to inform the eighth edition of the TNM classification of lung cancer. J Thorac Oncol 2014;9:1618-24.
- Travis WD, Brambilla E, Mu¨ller-Hermelink HK, et al. Pathology and genetics of tumours of the lung, pleura, thymus and heart. In: Travis WD, Brambilla E, Muller-Hermelink HK, et al. editors. World Health Organization Classification of Tumours. Lyon: IARC Press, 2004:9-124.
- Statistical Handbook of Japan 2020 Edited and Published by Statistics Bureau Ministry of Internal Affairs and Communications Japan. P20. Available online: https://www.stat.go.jp/english/data/handbook/pdf/2020all.pdf#page=23 (2021/12/23 accessed).
- Hong S, Moon YK, Park JK. Comparison of Surgical Outcomes and Survival between Octogenarians and Younger Patients after Pulmonary Resection for Stage I Lung Cancer. Korean J Thorac Cardiovasc Surg 2018;51:312-21. [Crossref] [PubMed]
- Nakao M, Ichinose J, Matsuura Y, et al. Outcomes after thoracoscopic surgery in octogenarian patients with clinical N0 non-small-cell lung cancer. Jpn J Clin Oncol 2020;50:926-32. [Crossref] [PubMed]
- Srisomboon C, Koizumi K, Haraguchi S, et al. Thoracoscopic surgery for non-small-cell lung cancer: elderly vs. octogenarians. Asian Cardiovasc Thorac Ann 2013;21:56-60. [Crossref] [PubMed]
- Mimae T, Saji H, Nakamura H, et al. Survival of Octogenarians with Early-Stage Non-small Cell Lung Cancer is Comparable Between Wedge Resection and Lobectomy/Segmentectomy: JACS1303. Ann Surg Oncol 2021;28:7219-27. [Crossref] [PubMed]
- Cannon NA, Iyengar P, Choy H, et al. Stereotactic ablative body radiation therapy for tumors in the lung in octogenarians: a retrospective single institution study. BMC Cancer 2014;14:971. [Crossref] [PubMed]
- Okami J. Treatment strategy and decision-making for elderly surgical candidates with early lung cancer. J Thorac Dis 2019;11:S987-97. [Crossref] [PubMed]
- Rivera C, Falcoz PE, Bernard A, et al. Surgical management and outcomes of elderly patients with early stage non-small cell lung cancer: a nested case-control study. Chest 2011;140:874-80. [Crossref] [PubMed]
- Detillon DDEMA, Veen EJ. Postoperative Outcome After Pulmonary Surgery for Non-Small Cell Lung Cancer in Elderly Patients. Ann Thorac Surg 2018;105:287-93. [Crossref] [PubMed]
- Hino H, Karasaki T, Yoshida Y, et al. Competing Risk Analysis in Lung Cancer Patients Over 80 Years Old Undergoing Surgery. World J Surg 2019;43:1857-66. [Crossref] [PubMed]
- Fukumoto K, Mori S, Shintani Y, et al. Impact of the preoperative body mass index on the postoperative outcomes in patients with completely resected non-small cell lung cancer: A retrospective analysis of 16,503 cases in a Japanese Lung Cancer Registry Study. Lung Cancer 2020;149:120-9. [Crossref] [PubMed]
- Prospective Studies Collaboration. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet 2009;373:1083-96. [Crossref] [PubMed]
- Zhang X, Liu Y, Shao H, et al. Obesity Paradox in Lung Cancer Prognosis: Evolving Biological Insights and Clinical Implications. J Thorac Oncol 2017;12:1478-88. [Crossref] [PubMed]
- Stefan N, Häring HU, Hu FB, et al. Metabolically healthy obesity: epidemiology, mechanisms, and clinical implications. Lancet Diabetes Endocrinol 2013;1:152-62. [Crossref] [PubMed]
- Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 2019;48:16-31. [Crossref] [PubMed]
- Takamori S, Tagawa T, Toyokawa G, et al. Prognostic Impact of Postoperative Skeletal Muscle Decrease in Non-Small Cell Lung Cancer. Ann Thorac Surg 2020;109:914-20. [Crossref] [PubMed]
- Kawaguchi Y, Hanaoka J, Ohshio Y, et al. Sarcopenia increases the risk of post-operative recurrence in patients with non-small cell lung cancer. PLoS One 2021;16:e0257594. [Crossref] [PubMed]
- Hino H, Saito T, Matsui H, et al. Utility of Geriatric Nutritional Risk Index in patients with lung cancer undergoing surgery. Eur J Cardiothorac Surg 2020;58:775-82. [Crossref] [PubMed]
- Yu R, Wong M, Leung J, et al. Incidence, reversibility, risk factors and the protective effect of high body mass index against sarcopenia in community-dwelling older Chinese adults. Geriatr Gerontol Int 2014;14:15-28. [Crossref] [PubMed]
Cite this article as: Hino H, Utsumi T, Maru N, Matsui H, Taniguchi Y, Saito T, Murakawa T. Surgical risk and survival impact of octogenarian lung cancer patients compared to those of younger patients undergoing surgery. Curr Chall Thorac Surg 2023;5:23.


