Titanium mesh in chest wall stabilization and reconstruction: a single center experience
Introduction
Intrathoracic organs and adequate respiratory activity are allowed by thoracic wall integrity and stability. Thoracic defects could be the result of a trauma, chest wall tumors removal or a locally-invasive tumor or metastasis (1). Chest wall tumors can be classified as benign or malignant, primary or secondary and from bone or soft tissue. Most patients present with a painful enlarging mass and surgical excision is frequently the only modality of treatment. Wide resection with tumor-free margins is necessary to minimize local recurrence and maximize the likelihood of long-term survival (2). The thoracic trunk is usually well vascularized and many tissues could be used for reconstructions, for example, local muscle flaps or greater omentum (useful to cover wounds) (3), but reconstruction with rigid prosthesis is required in moderate and large defects to improve chest wall stability and improve ventilation (4). Titanium results malleable but durable and these features make it adaptable to many types of chest wall defects and it could lead to restoring the original anatomical conformation with the consequent physiological benefits (3). Recently a new titanium mesh (MDF Medica) with more strength than synthetic meshes and high ductility and adaptability was proposed. We performed research in the English literature on Pubmed platform, using the words “Titanium chest wall”. We found a total of 48 articles and case reports were excluded. We present the results of our preliminary group of patients with a literature overview.
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Institutional Review Board of Ferrara S. Anna Hospital (No. CTOOB-3-F). Written informed consent was obtained from all patients or their legal guardians.
This report describes a single-center experience of six patients submitted to a reconstruction of the chest wall with the titanium mesh (MDF Medical s.rl.). After obtaining written informed consent from the patients for this study, we retrospectively recorded patient demographic data, comorbidities, type, and side of the defect, as well as the type of surgical procedure and postoperative outcomes, were analyzed. Between November 2015 from April 2018 six patients were enrolled, computerized tomography was obtained in all patients and a total body positron emission tomography (PET) scan has been implemented for correct staging in oncologic disease. Core needle biopsy, incisional biopsy or transbronchial biopsy were performed to identify neoplastic patients. General anesthesia with one-lung ventilation was used in planed lung procedure while for the other cases we proceeded with single lumen intubation. Lateral decubitus was the preferred position in the treatment of posterior or lateral thoracic defects, while the supine position was the choice way for anterior thoracic reconstruction. The oncological resection criteria have been always respected and 2.5 cm of macroscopic margin was the target in lung cancer involving the chest wall while at least 4 cm was required in a primary malignant tumor of the chest wall. Reconstruction of the chest wall in trauma patients was related to the clinical condition and a single incision close to fracture outbreaks is usually performed with greater muscle spared as possible. Non-oncological patient’ follow-up with a roentgenogram was performed in uncomplicated patients once a month for three months instead the computed tomography (CT) scan was reserved in case of unusual chest pain or fever disease. Oncological cases underwent CT scan follow up according to standard criteria for the disease. The mesh (MDF Medica S.r.l, Italy) is made of titanium, for medical use, grade 2. Prosthesis is available in two different thicknesses:
- 0.6 mm thick (used when greater rigidity is required for example sternal replacement or anterolateral huge defects.
- 0.4 mm thick used in cases of reconstructions requiring a more flexible material.
All these prostheses have the same size (20 cm × 14 cm) and their triangle design makes them non-deformable. Surgeons can adapt the mesh using mayo or heavier scissors. It is possible to fix this mesh using steel wires, thick polypropylene stitches, or screws through the triangular-shaped holes of the mesh.
Results
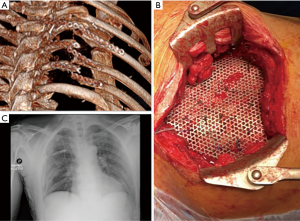
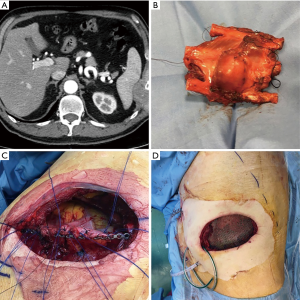
A total of 6 consecutive patients were enrolled in our institute from 2015 to 2018 (Table 1). The average age of patients was 57 years (range, 17–75 years). In three cases indications for surgery were primary chest wall tumors (Figure 1) and one case of a secondary tumor by lung cancer invading chest wall, the other three cases were a benign disease as trauma consequences (Figure 2). Frequently, localizations of chest wall defects were lateral (3 cases) and no sternal reconstruction was required. In one of these cases, titanium bars placed to treat a previous trauma were broken after a new car accident and they were replaced with a Mesh during operation. In five cases surgical operations were in elective time and in only one traumatic case it was an emergency. The average size of the defect was 31.6 cm2. In only one case a lung resection was performed due to advanced lung cancer. Two patients received titanium bars and titanium Mesh (one of this was the obese patient), others 4 cases were treated with mesh placement only. There was no materials rupture during surgery and the average operations time was 258 min (range, 210–310 min). Intensive care unit (ICU) required for 4 patients with a median of the length of ICU stay of 2.5 days (range, 0–21 days). The median length of hospital stay was 9.5 days (range, 5–32 days). Major complications occurred in only one case with a local infection in a patient with anamnesis of obesity (BMI 38.5), Asma and OSAS that was resolved with medical therapy. No perioperative deaths occurred.
Table 1
| Type of pathology | Size of defect (cm²) | Operation time (min.) | Intensive care unit | Length hospital of stay (days) |
|---|---|---|---|---|
| Trauma | 50 | 250 | Yes | 8 |
| Tumor | 54 | 310 | Yes | 11 |
| Trauma | 28 | 210 | Yes | 32 |
| Tumor | 10 | 220 | No | 5 |
| Trauma | 12 | 300 | No | 7 |
| Tumor | 36 | 260 | Yes | 12 |
| Average | 31.6 | 258 | – | – |
| Median (days) | – | – | 2.5 | 9.5 |
Discussion
In a large series of 200 patients reported by Mansour, the main indications for oncologic-surgery are primary lung cancer infiltrating the thorax or in case of lung cancer metastasis of the thoracic cage, primary chest wall tumors and primary breast cancer with recurrence or metastasis to the chest wall. Radio necrosis is rarer than in the past decades (3). Between the sternum and the anterior axillary line there is the area of anterior chest wall defects; lateral defects are between the anterior and posterior axillary lines, finally, posterior defects are located between the spine and posterior axillary line (5). Some of the indications for surgical treatment are untreatable pain; respiratory insufficiency; shifted, overlapping or impacted fractures, as well as deformity or instability of the sternum; hunched posture and limited movement of the trunk.
An unstable anterior chest wall represents an indication for surgery, as well as fracture displacement or a painful instability of more than seven days (6). Predictors of postoperative complications were identified by multivariate analysis: patient age, size of the chest wall defect, and concomitant anatomical lung resection (5).
The primary aim is to stabilize the chest wall, especially after extensive resections for tumors or severe deformities after trauma. This avoids paradoxical respiratory movement that leads to respiratory failure, lung hernia, and pain (7).
Chest wall resection and reconstruction for cancer are associated with high morbidity (range, 16–69%) (4), with respiratory insufficiency in as many as 27% of patients. The size of the chest wall defect results in the most significant predictor of complications by a multivariate analysis edited by Weyant et al. After reconstruction, the possible presence of flail segments leads to a reduction of the pulmonary toilets with a high incidence of respiratory complications and subsequently respiratory failure and the highest mortality rate. In the study published by Weyant et al., the highest mortality rate (44%) concerns cases of pneumonectomy combined with chest wall resection (5). Is it common opinion that larger defects than 5 cm in diameter or including more than 4 ribs should be reconstructed for the existence of a high risk of lung herniation and a reduced respiratory function, especially for anterolateral defects and full-thickness resections. Conversely, the scapula can protect from some apical-posterior defects, even 10 cm in size, with the exception of defects under the 4th rib posteriorly, with the tip of the scapula at risk to entrapment (1). The fix of a large chest wall defect can be also obtained with fewer ribs and rebuilding of each one does not appear necessary for chest stability. In cases of removal of 3 or 4 ribs, for example, chest cage stability can be obtained with a reconstruction of 2 ribs (8). In a review of about 200 patients who underwent chest wall surgery from 1975 to 2000 authors indicate a mean number of 4-2 ribs resected (3). To date, many techniques are described in the literature to stabilize a sternal fracture and recently several new surgical techniques and materials have been introduced for chest wall repair (7). However, clear guidelines on the management of chest wall diseases do not still exist. Wound complications, including surgical site infection (SSI), represent a serious problem in patients with rigid chest wall skeletal reconstruction, especially in immunocompromised patients for adjuvant chemo/radiotherapy (4). Many implant-related factors may influence susceptibility to local infection. These include the surface characteristics (Gristina 1987; Cordero, Munuera and Folgueira 1994), the technique and stability of fixation (Worlock et al. 1994), the size and shape of the implant (Melcher et al. 1994) and the material and its biocompatibility (Gerber and Perren 1980; Petty et al. 1985; Hierholzer and Hierholzer 1991) (9). It was observed that wound complications are more frequent in patients with higher BMI (Khalil et al.); however, it was statistically insignificant (4). Methyl methacrylate since the 1980s has been the main choice to reconstruct the sternum, ribs and chest wall, entirely or partially even because surgeons could prepare this type of mesh during operations. However methyl-methacrylate material cannot be permeable to fluids and this could lead to increase pain and excessive chest wall rigidity. Similar to methyl methacrylate, polytetrafluoroethylene (PTFE) is watertight and causes minimal foreign body reaction; however, it is also manageable. A 2 mm thick PTFE mesh is used to cover the chest wall defect using heavy permanent suture and chest wall reconstruction is obtained with a mesh as tight as possible. Its use is contraindicated in infected fields (1). Nowadays, the surgeon’s preference for prosthesis can still influence the choice of material. However, Deschamps et al. showed no significant difference in the rate of postoperative outcomes or complications after placing a Prolene mesh or a PTFE patch for chest wall reconstruction (3). The necessity to find a material that can be safe in the case of irradiated fields or local infections has led to the development of biological prostheses (Both human and porcine bioprosthetic materials). This type of meshes can incorporate into native tissue thanks also to revascularization and cellular repopulation with a consequent greater resistance to infections, but the main problem of this type of prosthesis remains the high cost (1). In the case of large defects, the use of autologous bone would involve a complex harvesting and additional aesthetic and functional insult in other body areas.
A bone-autograft or bone allograft has the same benefits against infection risk, compatibility, and host tissue incorporation, but the second one does not require any extra incisions or tissue removal for harvesting. Finally, tissue can be collected in a tissue-bank (10). Tissue reactions and bacterial adhesion are different depending on the choice of metal used. The ideal metal should have excellent adhesion characteristics to limit capsule formation, high tissue compatibility, have a minimal rate of corrosion and hypoallergenic (Perren 1991) (9). The first metal prostheses were used by a French surgeon in 1909 but after the year 1940 a spread of better-tolerated and easier materials, like plastic, modifying the modern era of chest wall reconstruction (1). More recently, the encouraging experiences with titanium implants in other fields of surgery convinced industries to produce titanium devices (plates, splints, and screws) for the reconstruction and stabilization of the thorax (Synthes®, STRATOSTM) and surgeons started to use titanium for reconstruction after demolition for tumor, to treat malformation, to fix sternal dehiscence-diastasis and fractures of the chest wall after trauma (11). A correct rigidity of the chest wall could be obtained with a 5-mm thickness mesh, avoiding endo-thoracic organ lesions and the inert condition of titanium could protect by infection (1). The optimal fixation is guarantee by screws placed in the bone bi-cortically and to use at least three screws for blocking each side of the bar (11). Since the introduction and use of titanium in thoracic surgery, a decrease of infectious (4%) and low rates (13%) of early implant rupture has been described and analyzed as being related to the technical specifications of titanium implants (12) but some authors support that best results are obtained with a composition of rigid biological or synthetic materials and soft tissue coverage (7). Our previous published data confirmed that after the demolition of the chest wall for tumors or in case of deformity of the chest wall, titanium meshes (MDF Medica) should be preferably associated with the use of meshes and muscle flaps (11). In retrospectively reviewed by Jean-Philippe Berthet et al. other different titanium systems (Stratos or the Matrix Fixation System) are analyzed in two European departments (between 2009 and 2013) and 54 patients are collected. There was a high rate of implant failure (44.4%, documented by postoperative CT scans) and all of them occurred within 14 months after the operation. Interestingly, deformations (anteroposterior bending) of the Stratos implants occurred in 22 patients (40.7%) and were associated with rupture in 13 cases. In contrast, no deformations occurred with the Matrix system. Most failures (87%) are asymptomatic and the anterior location of titanium chest wall osteosynthesis (TCWO) was indicated as a significant risk factor. In another experience by Fabre and colleagues, the authors did not observe any failure. However, radiologic follow-up after TCWO is focused on evidence of tumor recurrence, not on TCWO systematically performed in for pectus repair (12). Finally, in one case Adli Azam et al. observed layers of fibrous tissue close the titanium plates which were not well documented in the previous series. It developed as early as two weeks after the placement of the titanium mesh. Authors described this layer as a ‘pseudopleura’, which provided protection for direct entry to the pleural cavity and the reasons for the formation of these layers remain unknown. It could have been due to the inflammatory reaction secondary to infection or due to the presence of the titanium mesh itself, which allowed granulation tissue to form. New studies on this subject will be needed (2). Recently, the help provided by new devices such as 3D-printers and computed based navigation surgery seems to simplify preoperative planning and improve the selection of the allograft bone and the recipient’s chest wall (1). The tridimensional custom-made titanium-printed prosthesis can represent a safe alternative to traditional materials for chest wall reconstruction. However, current main limitations are uncomfortable intraoperative placement, long manufacturing time or higher costs than other standard devices and the use still remains quite limited (13).
In conclusion, based on our experience, titanium meshes (MDF Medica) can perform an optimal chest wall stabilization and reconstruction in neoplastic, traumatic and malformation, with low morbidity, no postoperative mortality, acceptable operating time and postoperative hospital stay. We obtained long-term restoration of a normal respiratory function, with acceptable plates related morbidity and chest pain.
It is necessary to perform the largest studies to better standardize the use of this titanium prosthesis, with the purpose of reducing complications and improve results in terms of respiratory function, chest pain and quality of life.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Zaraca, Reinhold Perkmann, Luca Bertolaccini and Roberto Crisci) for the series “Thoracic Surgery Without Borders” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts.2020.01.02/coif). The series “Thoracic Surgery Without Borders” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This retrospective study was approved by the Institutional Review Board of Ferrara S. Anna Hospital (No. CTOOB-3-F). Written informed consent was obtained from all patients or their legal guardians.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sanna S, Brandolini J, Pardolesi A, et al. Materials and techniques in chest wall reconstruction: a review. J Vis Surg 2017;3:95. [Crossref] [PubMed]
- Adli Azam MR, Raja Amin RM. Huge chest wall tumour resection and reconstruction using titanium mesh. Malays J Med Sci 2015;22:70-3. [PubMed]
- Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: A 25-year experience. Ann Thorac Surg 2002;73:1720-5; discussion 1725-6.
- Khalil HH, Malahias MN, Balasubramanian B, et al. Multidisciplinary oncoplastic approach reduces infection in chest wall resection and reconstruction for malignant chest wall tumors. Plast Reconstr Surg Glob Open 2016;4:e809. [Crossref] [PubMed]
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- Schulz-Drost S, Oppel P, Grupp S, et al. Surgical fixation of sternal fractures: Preoperative planning and a safe surgical technique using locked titanium plates and depth limited drilling. J Vis Exp 2015;e52124. [PubMed]
- Tamburini N, Grossi W, Sanna S, et al. Chest wall reconstruction using a new titanium mesh: a multicenters experience. J Thorac Dis 2019;11:3459-66. [Crossref] [PubMed]
- Iarussi T, Pardolesi A, Camplese P, et al. Composite chest wall reconstruction using titanium plates and mesh preserves chest wall function. J Thorac Cardiovasc Surg 2010;140:476-7. [Crossref] [PubMed]
- Arens S, Schlegel U, Printzen G, et al. Influence of materials for fixation implants on local infection. An experimental study of steel versus titanium DCP in rabbits. J Bone Joint Surg Br 1996;78:647-51. [Crossref] [PubMed]
- Dell’Amore A, Cassanelli N, Dolci G, et al. An alternative technique for anterior chest wall reconstruction: The sternal allograft transplantation. Interact Cardiovasc Thorac Surg 2012;15:944-7. [Crossref] [PubMed]
- de Palma A, Sollitto F, Loizzi D, et al. Chest wall stabilization and reconstruction: Short and long-term results 5 years after the introduction of a new titanium plates system. J Thorac Dis 2016;8:490-8. [Crossref] [PubMed]
- Berthet JP, Gomez Caro A, Solovei L, et al. Titanium implant failure after chest wall osteosynthesis. Ann Thorac Surg 2015;99:1945-52. [Crossref] [PubMed]
- Aranda JL, Novoa N, Jiménez MF. Thoracic customized modular titanium-printed prosthesis. AME Case Reports 2019;3:35. [Crossref] [PubMed]
Cite this article as: Maniscalco P, Fabbri N, Quarantotto F, Tamburini N, Cavallesco G. Titanium mesh in chest wall stabilization and reconstruction: a single center experience. Curr Chall Thorac Surg 2020;2:13.