State of the art and operative technique for spray cryotherapy for central airway obstruction (CAO)—a narrative review
Introduction
Central airway obstruction (CAO) is a debilitating disorder that can result from a wide range of conditions. Common etiologies include iatrogenic trauma (post tracheotomy or intubation), neoplastic disease, collagen vascular disease (systemic lupus erythematosus, granulomatosis with polyangiitis and sarcoidosis), tuberculosis, post lung transplant stenosis and idiopathic strictures (1-4).
The causes of CAO may be broadly classified as benign and malignant. Foreign body aspiration, tracheobronchomalacia, and tracheal strictures due to tracheostomy tube placement are all benign causes of stenosis. Less common causes include airway papilloma (5), endobronchial hamartoma (6), and laryngocele (7). Malignant causes include primary lung cancer (8,9), primary tumors of the airway other than lung cancer, and metastatic disease. The underlying etiology dictates the mechanism of obstruction. Tumors cause intrinsic or extrinsic compression, while trauma, inflammation, and infection may result in granulation tissue, calcium deposits, and intramural edema. Finally, disorders of airway cartilage result in wall thinning and subsequent collapse.
The vast majority of these stenotic lesions can be addressed with bronchoscopic ablative modalities coupled with mechanical dilation or stenting. There has been a paradigm shift from open surgery to minimally invasive endoluminal techniques for the management of CAO in recent years (10). This is particularly true in patients with long strictures, multiple comorbidities, diffuse inflammatory conditions, recurrent disease or disease involving the cricoid cartilage or sub-glottis where open resection would endanger the recurrent nerves.
Traditionally, heat-based modalities such as electrocautery, lasers and argon plasma coagulation have been used in conjunction with mechanical dilation and the results have generally been satisfactory. However, there is concern that the use of heat leads to future additional scarring and hence while effective in the short term, may lead to extension of the stenosis (11,12). Heat based modalities also carry a fire hazard and require patients to tolerate a FiO2 of less than 40% (13,14). The risk of ignition also limits their use in proximity to airway implants such as stents (13,14). Airway stents, on the other hand, though convenient and easy to deploy, come with their own set of complications such as granulation tissue formation, neo-epithelialization, stent migration and mucus plugging (15). There is a need, therefore to explore more effective alternative therapies for CAO.
Cryotherapy is the process of using extreme cold to destroy unwanted tissue. It was first used on an endobronchial tumor in 1968 by Gage (16). Initial technology included contact dependent cryoprobe -based therapy that utilized the Joule-Thompson principle in which rapid release of a compressed gas through a narrow aperture achieves a concomitant drop in temperature. This contact probe cryotherapy allows for a delayed cytotoxic effect by direct application as well as immediate relief by cryodebulking where the cryoprobe is adhered to the obstructing mass and is then firmly pulled away removing large pieces of tissue. This is often a tedious and time-consuming process due to the surface area limitations of the cryoprobe (17).
Spray cryotherapy (SCT) is a more recent application of cryotherapy that has now been adopted at many centers. It obviates the need for contact with target tissue. It utilizes a 7-French catheter to spray liquid nitrogen (the cryogen) via the working channel of an endoscope to cause tissue ablation by flash freezing at −196 ℃. One to two cycles of cryospray for 5 seconds are followed by a one-minute thaw period. SCT allows a linear distribution of cryogen over a larger area as opposed to the cryoprobe that allows cooling in a radial fashion from the point of contact only (12,13). It bypasses many of the limitations of traditional heat-based modalities and has been shown to have a hemostatic and analgesic effect (18). SCT does not have a fire risk (19). Herein we review current literature on SCT in the airway and describe our own experience using this technique. We present this article in accordance with the Narrative Review reporting checklist (available at https://ccts.amegroups.com/article/view/10.21037/ccts-21-12/rc).
Methods
We conducted an electronic-based literature search on PubMed and Google Scholar databases of English language publications to identify data on utility of SCT for CAO (Table 1). The time frame of our search included all studies published up to January 2021. The following medical subject heading terms, keywords and their combinations were used: “Spray cryotherapy; SCT; Airway; Central airway obstruction”. Only articles that were available as full text articles were included in our review. We also manually searched the reference lists of relevant studies.
Table 1
| Items | Specification |
|---|---|
| Date of search | March 1st 2021 |
| Databases and other sources searched | PubMed, Google Scholar |
| Search terms used | Spray cryotherapy; SCT; airway; central airway obstruction |
| Timeframe | All studies published prior to January 2021 |
| Inclusion criteria | Full text articles, English language articles |
| Selection process | Authors MZB, JW and ZM conducted literature review. All authors agreed on final list of included studies. |
| Additional considerations | Manual search of reference lists of articles |
SCT, spray cryotherapy.
Discussion
Cryotherapy causes the formation of extracellular and intracellular ice crystals that induce cell death
Cryotherapy employs the Joule-Thompson effect where rapid expansion of a gas from high to low pressure leads to a dramatic drop in temperature (20). This application of cold energy in tissue leads to the formation of ice crystals by freezing intracellular and extracellular water. Contact probe cryotherapy leads to a slow cooling down to –40 ℃ and causes mainly extracellular ice which leads to cell death by electrolyte imbalance and dehydration (18). This is in contrast to SCT that rapidly reduces tissue temperature to –196 ℃ and causes instantaneous freezing of tissue by the formation of intracellular ice crystals resulting in cell death by damaging cellular machinery, in particular the mitochondria (18,20). For benign strictures that are acellular, a different mechanism is implicated. It is known from previous dermatology literature that cryotherapy induces connective tissue remodeling and this may allow for a more easily dilated stricture (18). Other processes that play a role in the therapeutic effect of SCT include local vasoconstriction, thrombosis as well as immune mediated damage (21).
SCT demonstrates preservation of extracellular matrix (ECM) that may enhance subsequent healing
Collagen, cartilage and tissues with a poor vascular supply and low water content are known to show relative resistance to cryotherapy. Therefore, the ECM is usually preserved and hence leads to healing with minimal subsequent scarring (20). In a recent small animal experiment, the histological changes observed after SCT were enunciated (22). Six Yorkshire pigs underwent evaluation with flexible bronchoscopy, endoscopy and thoracotomy following SCT of the airway, esophagus and other intrathoracic structures. In all structures, the ECM was well preserved. This was in sharp contrast to the protein denaturation that occurs with heat-based modalities. It is hypothesized that the preservation of ECM may be the histological basis of low rates of scarring as it provides a structural scaffolding for wound healing (12,22). Reduced scarring may also allow for a decreased number of re-interventions as well as prolong the symptom free interval between interventions (12). It is also reported that following cryotherapy, underlying tissue may become more malleable and this may allow subsequent scar remodeling and regeneration of normal tissue architecture (12).
Early experience with the initial SCT device demonstrated promising outcomes
SCT was initially used to treat esophageal conditions such as Barret’s esophagus, dysplasia and carcinomas (12,17). Studies have shown that SCT eradicated 97% of high-grade dysplasia in Barret’s esophagus and had a complete response of intraluminal disease in 72% patients with T1 esophageal cancers (22-24). These encouraging long term outcomes led to investigators extending this modality to the airway. In the initial report published, SCT was performed on 21 lung cancer patients using the Cryospray Ablation System (CSA Medical, Inc., Baltimore, Md) (17). All patients in the study achieved regeneration of histologically normal epithelium. Post procedural mean pain score on the visual analog scale (VAS) was 2.9. There were no adverse effects of treatment.
In another study, SCT was utilized to manage three patients with glottic and subglottic stenosis secondary to neck radiation, prior tracheostomy and idiopathic causes. Luminal patency with minimal blood loss, some degree of mucosal normalization and restoration of laryngeal function was achieved in all three patients (25).
Fernando et al. also demonstrated efficacy of SCT in 35 patients who developed CAO (19). Airway narrowing was graded on a four-tier grading system based on the degree of lumen involved. Follow up bronchoscopy in 28 patients demonstrated a significant improvement in airway narrowing from an initial grading score of 3.5 to 2.03 (P<0.001). Seventeen (49%) patients required reinterventions. Twelve (36.4%) patients reported complete resolution of symptoms whereas sixteen (48.5%) patients had improved symptoms. There were only two complications reported namely pneumothorax (n=1) and subglottic edema requiring intraoperative tracheostomy (n=1).
Despite these encouraging results, a few studies reported complications with the early off label use of SCT for CAO, because of which the SCT device underwent a redesign. These included hemodynamic events such as bradycardia, hypotension, tachycardia, desaturations, ST segment changes and airway tears as well as barotrauma induced effects such as pneumothorax and pneumomediastinum (13,14). In 2008, a clinical trial was initiated (NCT00747461) to investigate the role of SCT in benign airway disease. This trial was terminated prematurely due to adverse effects. These studies were all done using the older generation cryospray device and it was suspected that complications arose due to inadequate egress of nitrogen gas as well as the flow characteristics of the older device. Consequently, several modifications were made and in 2012 the advanced truFreeze device with adjustable delivery and flow rates gained US Food and Drug Administration (FDA) clearance for use in the airway.
Studies with the latest generation truFreeze device have shown good outcomes with low complication rates
As mentioned above, SCT works on the principle of flash freezing unwanted tissue by spraying liquid nitrogen. These liquid nitrogen droplets then transform into gas in the endoluminal space and need to be evacuated in order to prevent barotrauma. In the gastrointestinal tract, this can be achieved through active suctioning. This is not a practical option in the airway as there is less room for additional tubes and the active suction may actually end up removing oxygen from the patient’s lungs. Therefore, gas evacuation in the airway is primarily achieved passively through the mouth via pressure differentials.
The newer generation truFreeze device comes with an adjustable flow rate. The previous device delivered liquid nitrogen at only 25 watts of energy (normal flow). The current device, on the other hand comes with a low flow rate setting that delivers liquid nitrogen at 12.5 watts of energy. This allows for a slower buildup of nitrogen gas and gives more time to identify and manage nitrogen trapping.
The first report utilizing this new device described four patients with CAO due to lung cancer and prior intubation (26). Patency of the airway was achieved in all patients and there were no reported complications. This report was soon followed by another study that described an institutional experience in 27 patients who underwent 80 SCT sessions (27). Of these, 18 (22.5%) were done with the previous device and 62 (77.5%) were done with the newer system. Only three complications (all transient hypoxia) were encountered and only one of these was with the new device.
We have also had success using the new truFreeze cryospray. Our group has previously published data on 26 patients with benign CAO treated at a tertiary care hospital (10). Thirteen patients had CAO secondary to granulomatosis with polyangiitis, 8 had prior intubation and 5 patients had idiopathic strictures. We reported a median follow up period of 11 months (range, 1–26 months) There were no significant intraoperative or postoperative complications encountered and all our patients had improvement in symptoms. We observed a mean change of 1.39 in grade of stenosis (P<0.001). Our study was important as it demonstrated the efficacy of SCT in patients with recalcitrant stenosis. Our cohort was a unique group as the majority of patients had previously shown poor response with other endoluminal therapies.
We recommend the procedure being done using suspension laryngoscopy. We feel that is the safest approach in terms of delivery of SCT. Laryngeal suspension is utilized by some head and neck surgeons and is an important technique for the thoracic surgeon s and interventional pulmonologists to be familiar with. We believe it allows for the most effective egress of nitrogen gas and hence adds great safety to this procedure. Our technique involves only the treatment of larger central airways. We sometimes prime the catheter outside the patient and deliver the SCT 5 seconds after the appearance of frost through the working channel of a therapeutic bronchoscope. We ensure adequate egress which is confirmed visually and as described our technique provides for a wide-open airway. And hence allows for maximum egress of gas. We typically deliver 3–4 cycles of SCT interspaced with endoscopic balloon dilation. It is possible and safe to perform the procedure using an endotracheal tube however at a minimum 8.5 size endotracheal tube should be used with the cuff completely deflated and the circuit disconnected during delivery of SCT. The takeaway lessons in our over 250 procedures with minimal complications is to ensure complete egress of gas.
In addition, if there is any concern regarding egress then a low flow setting can be employed. It is also important to work with an anesthesiologist who is familiar with the technique and of possible complications.
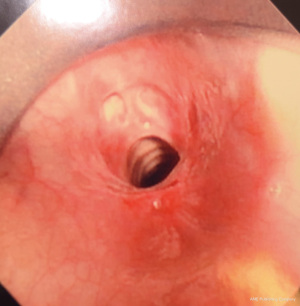
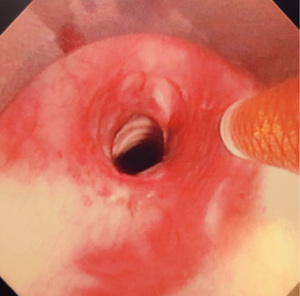
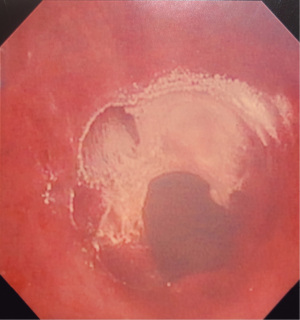
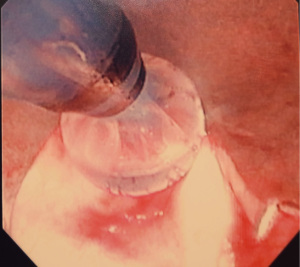
Figures 1-5 and Video 1 demonstrate our technique using the truFreeze device to treat CAO.
Summary and conclusions
SCT is an advanced bronchoscopic technique with the potential to adequately treat benign and malignant airway strictures. It maintains the integrity of underlying ECM which allows for a more controlled wound healing response, thereby encouraging scar remodeling and tissue regeneration. Several recent studies have shown good outcomes with this therapy. Based on our own experience and current data, we believe SCT may be particularly useful in patients who have had treatment failure with conventional modalities. A prospective multicenter registry is currently underway (NCT01802203) and is expected to be completed soon. This should provide additional information on the utility and efficacy of SCT for CAO.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://ccts.amegroups.com/article/view/10.21037/ccts-21-12/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-21-12/coif). FYB serves as an unpaid editorial board member of Current Challenges in Thoracic Surgery from October 2012 to September 2023. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Murgu SD, Egressy K, Laxmanan B, et al. Central Airway Obstruction: Benign Strictures, Tracheobronchomalacia, and Malignancy-related Obstruction. Chest 2016;150:426-41. [Crossref] [PubMed]
- Galluccio G, Lucantoni G, Battistoni P, et al. Interventional endoscopy in the management of benign tracheal stenoses: definitive treatment at long-term follow-up. Eur J Cardiothorac Surg 2009;35:429-33; discussion 933-4. [Crossref] [PubMed]
- Nouraei SA, Obholzer R, Ind PW, et al. Results of endoscopic surgery and intralesional steroid therapy for airway compromise due to tracheobronchial Wegener's granulomatosis. Thorax 2008;63:49-52. [Crossref] [PubMed]
- Barros Casas D, Fernández-Bussy S, Folch E, et al. Non-malignant central airway obstruction. Arch Bronconeumol 2014;50:345-54. [PubMed]
- Blackledge FA, Anand VK. Tracheobronchial extension of recurrent respiratory papillomatosis. Ann Otol Rhinol Laryngol 2000;109:812-8. [Crossref] [PubMed]
- Cosío BG, Villena V, Echave-Sustaeta J, et al. Endobronchial hamartoma. Chest 2002;122:202-5. [Crossref] [PubMed]
- Kaya G, Ladas A, Howlett D. Laryngocele causing airway obstruction. BMJ 2016;352:i1368. [Crossref]
- Chhajed PN, Eberhardt R, Dienemann H, et al. Therapeutic bronchoscopy interventions before surgical resection of lung cancer. Ann Thorac Surg 2006;81:1839-43. [Crossref] [PubMed]
- Cosano Povedano A, Muñoz Cabrera L, Cosano Povedano FJ, et al. Endoscopic treatment of central airway stenosis: five years' experience. Arch Bronconeumol 2005;41:322-7. [Crossref] [PubMed]
- Bhora FY, Ayub A, Forleiter CM, et al. Treatment of Benign Tracheal Stenosis Using Endoluminal Spray Cryotherapy. JAMA Otolaryngol Head Neck Surg 2016;142:1082-7. [Crossref] [PubMed]
- Qiu XJ, Zhang J, Wang T, et al. Nonstent Combination Interventional Therapy for Treatment of Benign Cicatricial Airway Stenosis. Chin Med J (Engl) 2015;128:2154-61. [Crossref] [PubMed]
- Fernando HC, Dekeratry D, Downie G, et al. Feasibility of spray cryotherapy and balloon dilation for non-malignant strictures of the airway. Eur J Cardiothorac Surg 2011;40:1177-80. [Crossref] [PubMed]
- Finley DJ, Dycoco J, Sarkar S, et al. Airway spray cryotherapy: initial outcomes from a multiinstitutional registry. Ann Thorac Surg 2012;94:199-203; discussion 203-4. [Crossref] [PubMed]
- Pedoto A, Desiderio DP, Amar D, et al. Hemodynamic Instability Following Airway Spray Cryotherapy. Anesth Analg 2016;123:1302-6. [Crossref] [PubMed]
- Bhora F, Baig MZ. Is Long-Term Stenting for Benign Airway Obstruction Effective. In: Ferguson MK. Editor. Difficult Decisions in Thoracic Surgery. UK: Springer-Verlag, 2020.
- DiBardino DM, Lanfranco AR, Haas AR. Bronchoscopic Cryotherapy. Clinical Applications of the Cryoprobe, Cryospray, and Cryoadhesion. Ann Am Thorac Soc 2016;13:1405-15. [Crossref] [PubMed]
- Krimsky WS, Broussard JN, Sarkar SA, et al. Bronchoscopic spray cryotherapy: assessment of safety and depth of airway injury. J Thorac Cardiovasc Surg 2010;139:781-2. [Crossref] [PubMed]
- Moore RF, Lile DJ, Abbas AE. Current status of spray cryotherapy for airway disease. J Thorac Dis 2017;9:S122-9. [Crossref] [PubMed]
- Fernando HC, Sherwood JT, Krimsky W. Endoscopic therapies and stents for benign airway disorders: where are we, and where are we heading? Ann Thorac Surg 2010;89:S2183-7. [Crossref] [PubMed]
- Sachdeva A, Pickering EM, Lee HJ. From electrocautery, balloon dilatation, neodymium-doped:yttrium-aluminum-garnet (Nd:YAG) laser to argon plasma coagulation and cryotherapy. J Thorac Dis 2015;7:S363-S379. [PubMed]
- Chaddha U, Hogarth DK, Murgu S. Bronchoscopic Ablative Therapies for Malignant Central Airway Obstruction and Peripheral Lung Tumors. Ann Am Thorac Soc 2019;16:1220-9. [Crossref] [PubMed]
- Au JT, Carson J, Monette S, et al. Spray cryotherapy is effective for bronchoscopic, endoscopic and open ablation of thoracic tissues. Interact Cardiovasc Thorac Surg 2012;15:580-4. [Crossref] [PubMed]
- Greenwald BD, Dumot JA, Abrams JA, et al. Endoscopic spray cryotherapy for esophageal cancer: safety and efficacy. Gastrointest Endosc 2010;71:686-93. [Crossref] [PubMed]
- Shaheen NJ, Greenwald BD, Peery AF, et al. Safety and efficacy of endoscopic spray cryotherapy for Barrett's esophagus with high-grade dysplasia. Gastrointest Endosc 2010;71:680-5. [Crossref] [PubMed]
- Krimsky WS, Rodrigues MP, Malayaman N, et al. Spray cryotherapy for the treatment of glottic and subglottic stenosis. Laryngoscope 2010;120:473-7. [Crossref] [PubMed]
- Browning R, Parrish S, Sarkar S, et al. First report of a novel liquid nitrogen adjustable flow spray cryotherapy (SCT) device in the bronchoscopic treatment of disease of the central tracheo-bronchial airways. J Thorac Dis 2013;5:E103-6. [PubMed]
- Browning R, Turner JF Jr, Parrish S. Spray cryotherapy (SCT): institutional evolution of techniques and clinical practice from early experience in the treatment of malignant airway disease. J Thorac Dis 2015;7:S405-14. [PubMed]
Cite this article as: Baig MZ, Weber JF, Muslim Z, Al Shetawi AH, Bhora FY. State of the art and operative technique for spray cryotherapy for central airway obstruction (CAO)—a narrative review. Curr Chall Thorac Surg 2023;5:32.