Anastomotic complications after lung transplantation
Introduction
Since the first lung transplant performed by James Hardy in 1963 in the University of Mississippi, there has been significant progress in the surgical techniques and perioperative care. However, the median survival of lung transplant recipients continue to be poor compared to other transplanted organs. While infections and graft failure account for approximately 35% of deaths in the first year after transplantation, the development of different forms of chronic lung allograft dysfunction and neoplasms, account for the majority of late deaths. Anastomotic complications are rare, but still are associated to significant postoperative problems that should be appropriately managed.
The lung transplant technique comprises three anastomoses: the bronchial, the pulmonary artery and the venous anastomosis. In the present review, we will update the complications arising in these anastomoses focusing in their diagnosis and management options.
Airway complications
In the early lung transplant era, airway complications were frequent and associated to high morbidity and mortality rates (1). More recently, improvements in surgical technique, organ preservation and immunosuppressive therapy, have led to a significant reduction of these complications. Nonetheless, in the last two decades, the incidence of airway complications continues to be around 2% to 8% in large series (2-4). In our experience, the rate of bronchial complications in a series of 343 anastomoses was 9% with no differences in long-term survival (5).
This variability is attributed to the disperse criteria to define airway complications. Although different classification systems have been proposed, none of them have been accepted worldwide (6-8). Recently, the International Society for Heart and Lung Transplantation (ISHLT) has proposed a new classification aimed at standardizing the endoscopic findings (Table 1), based on the type of complication, its location, and its extent throughout the airway. This proposed grading system is based on the early bronchoscopic findings after the lung transplant (9).
Table 1
| Ischemia and necrosis (I) |
| Location |
| a. Perianastomotic—within 1 cm of anastomosis |
| b. Extending >1 cm from anastomosis to major airways (bronchus intermedius and distal left main-stem) |
| c. Extending >1 cm from anastomosis into lobar or segmental airways |
| Extent |
| a. <50% circumferential ischemia |
| b. >50% to 100% circumferential ischemia |
| c. <50% circumferential necrosis |
| d. >50% to 100% circumferential necrosis |
| Dehiscence (D) |
| Location |
| a. Cartilaginous |
| b. Membranous |
| c. Both |
| Extent |
| a. 0% to 25% of circumference |
| b. >25% to 50% of circumference |
| c. >50% to 75% of circumference |
| d. >75% of circumference |
| Stenosis (S) |
| Location |
| a. Anastomotic |
| b. Anastomotic plus lobar/segmental |
| c. Lobar/segmental only |
| Extent |
| a. 0% to 25% reduction in cross-sectional area |
| b. >25% to 50% reduction in cross-sectional area |
| c. >50% but <100% reduction in cross-sectional area |
| d. 100% obstruction |
| Malacia (M) |
| Location |
| a. Perianastomotic—within 1 cm of anastomosis |
| b. Diffuse—involving anastomosis and extending beyond 1 cm |
ISHLT, International Society for Heart and Lung Transplantation.
Etiology
The bronchial anastomosis performed in a lung transplant has no blood supply to promote an adequate healing. In the standard lung transplant technique, the bronchial arteries are transected and not anastomosed, therefore, the bronchial anastomosis is initially dependent solely on the retrograde pulmonary blood flow coming from a poorly oxygenated pulmonary arterial system. After 2–4 weeks from the completion of the lung transplant, this retrograde blood flow is substituted by a systemic revascularization of the donor airway by the recipient bronchial circulation (10). Therefore, those conditions decreasing pulmonary blood flow or increasing the pulmonary vascular resistance leads to airway ischemia and compromise the bronchial healing (Table 2).
Table 2
| Poor graft preservation |
| Lung ischemia-reperfusion injury |
| Severe edema |
| Rejection |
| Infection |
| Inflammation |
| Prolonged positive pressure ventilation |
| Excessive length of donor bronchus |
Bronchial complications derived from donor airway ischemia are interrelated and one complication may lead to another with time. Initially presents with some degree of mucosal necrosis that may progress to involve the full-thickness of the bronchial wall and produce an anastomotic dehiscence. Later on, these problems result in fibrotic changes with different degrees of airway stenosis, granulation tissue formation, and compromised structural integrity of the airway with malacia (11).
Risk factors
A summary of risk factors associated to the development of airway complications after lung transplantation is presented in Table 3.
Table 3
| Donor length of mechanical ventilation |
| Donor/recipient height mismatch |
| Brain-death donors |
| Recipient low cardiac output |
| Right-sided bronchial anastomoses |
| Double lung transplantation |
| Poor preservation techniques |
| Primary graft dysfunction |
| Recipient mechanical ventilation |
| Airway infections |
| Immunosuppression |
| Transplantation for cystic fibrosis |
| Surgical technique |
Several donor-related factors have been reported to be associated to anastomotic bronchial complications after a lung transplant: prolonged donor’s mechanical ventilation, donor/recipient height mismatch, and brain-dead donors (as opposed to donors after circulatory death) (12,13).
The side and type of lung transplant may also play a role. Right-sided bronchial anastomoses have been associated to higher risk of complications than left ones, probably related to the anatomical perfusion differences between both main bronchi (the right bronchus perfused by a one bronchial artery and the left bronchus receiving two bronchial arteries) (2). In our experience, double lung transplants were also associated to airway complications. In a series of 214 lung transplants, we observed that from 27 patients with bronchial complications, 23 were double lung transplants, with a 7.4-fold risk for the development of bronchial complications than single lung transplants. We suggested that the need of the retrograde collateral blood flow to be distributed into both grafts might make them more prone to develop airway complications (11).
A deficient organ preservation technique may compromise bronchial healing by decreasing the retrograde bronchial perfusion, leading to endothelial edema and acute reperfusion lung injury (14). On one hand, the use of low-potassium dextran preservation solutions with the addition of glucose has led to successful extended 12-hour lung preservation times (15). On the other hand, the technique of administering the preservation solution is also important in decreasing the incidence of bronchial complications. We could demonstrate the benefits of a double antegrade and retrograde administration of the preservation solution to improve the function of the graft at the early stages (16). This dual perfusion in the donor has been subsequently adopted in the majority of lung transplant centers worldwide. In addition, patients developing post-transplant primary graft dysfunction (PGD), are also at increased risk of airway ischemia. These patients develop diffuse alveolar damage and increased vascular permeability resulting in interstitial edema and reduction of pulmonary blood flow (17).
Postoperative recipient mechanical ventilation has also been related with bronchial ischemia. It has been suggested that high levels of positive end-expiratory pressure (PEEP), may compromise the bronchial anastomosis healing by interfering the bronchial mucosal blood flow (4,5,10,11). On the contrary, some authors have defended a beneficial effect of PEEP in promoting a more effective retrograde collateral blood flow to reach the bronchial anastomosis (18). In our early experience, mechanical ventilation was associated with airway complications (11). In a more recent analysis of our data, we observed that long postoperative mechanical ventilation predicted airway complications, with a 3.5-fold risk higher than those recipients being weaned from the ventilator early after the lung transplant (5). Probably, it is not the mechanical ventilation itself but factors related to prolonged postoperative ventilation requirements those really related with the development of airway complications. These factors include PGD, infections, or hemodynamic instability, among others.
Infections in the airways are also related to the development of bronchial complications. This is especially true for fungal infections by Aspergillus, Candida, Rhizopus, and Mucor species (4,19). Therefore, the appropriate treatment of post-transplant airway infections may also reduce the incidence of bronchial anastomotic complications. In our experience, patients developing post-transplant bronchial complications were frequently colonized by gram negative microorganisms (Pseudomonas cepacia), Aspergillus, or presented cytomegalovirus (CMV) infection and/or disease. In our series, recipients with a colonized airway had a 3.2-fold increased risk of developing bronchial complications than those without bronchial colorizations (5).
Some immunosuppressive agents, such as sirolimus, severely impairs airway healing increasing the rates of catastrophic anastomotic complications after the lung transplant (20). For this reason, the current recommendation is to avoid the use of sirolimus until a complete healing of the bronchial anastomosis. On the other hand, the use of corticosteroids in the preoperative period is no longer a major concern regarding the bronchial anastomosis healing. In fact, it has been demonstrated that the steroid use is associated to less granulation tissue formation and longer post-transplant survival (7,21). In our experience, low doses of preoperative steroids were not associated to the development of bronchial complications (5,11).
We have observed that almost half of airway complications appear in transplants for cystic fibrosis (5,11). This association has been reported previously by other authors (22) with rates of bronchial stenosis in these patients around 24%. Unfortunately, to date, there is no a clear explanation of such increased rates of airway complications in cystic fibrosis patients.
The technique of bronchial anastomosis has been a major concern as a potential risk factor of airway complications after lung transplantation. Although a telescoping technique was defended in the early years (7), the current evidence favors the use of an end-to-end bronchial anastomosis in minimizing the incidence of airway complications (4,12), and has gained widespread use. In general, whenever possible, a direct end-to-end technique is preferred (23,24), but, at present, there is no a definitive advantage of one anastomotic technique over another in the literature. Additionally, different procedures have been described to reduce the incidence of bronchial complications, such as keeping the donor bronchus as short as possible and wrapping the anastomosis with vascularized pedicles (7). Also, some transplant centers perform direct anastomosis of the bronchial arteries, in an effort to reduce the bronchial anastomosis ischemia (25), however, this technique requires the use of cardiopulmonary bypass, and prolongs the transplant procedure, and there is no evidence of a clear benefit of this technique over the standard technique.
Diagnosis and management
Necrosis and dehiscence
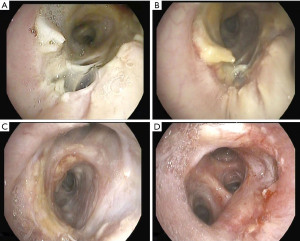
Bronchial dehiscence is a severe complication which is associated with high mortality rates (10). As commented above, dehiscence is the consequence of the progression of a mucosal necrosis that usually occurs within the first 1–4 weeks after transplantation. Some degree of mucosal necrosis is usually seen after lung transplantation, but disappear gradually when mucosal revascularization occurs. However, in some instances, this necrosis progresses to a partial or, occasionally, a complete anastomotic dehiscence. The reported incidence is between 1% and 10% (4,5,10). In our experience, in a review of 343 bronchial anastomoses, 5 partial dehiscence were diagnosed in four patients within the first month post-transplant (1.4% of anastomoses) (11). No patients in these series presented a complete dehiscence. Complete dehiscence has high mortality due to sepsis and ventilatory problems: inability to wean from mechanical ventilation, and lung collapse despite proper drainage of pleural spaces.
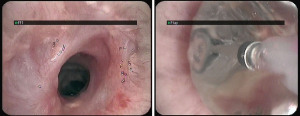
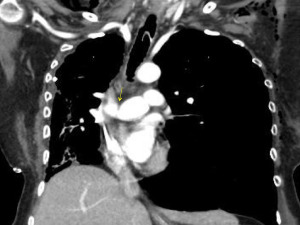
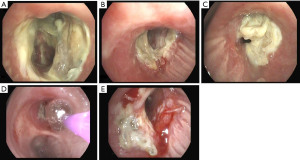
Dehiscence is diagnosed by chest CT scan in which bronchial wall defects, bronchial narrowing and extraluminal air, may be seen (26) (Figures 1,2). In general, dehiscence less than 4 mm heal spontaneously without need of invasive procedures, on the contrary, larger defects will require individualized treatment. The definitive diagnosis requires a flexible bronchoscopy, because the CT scan does not provide an accurate information regarding the status of the bronchial mucosa in terms of degree of necrosis that might anticipate a bronchial dehiscence (9,10,26).
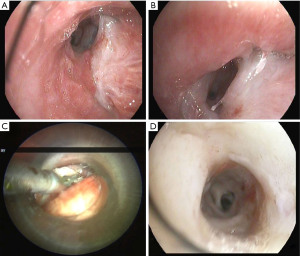
The treatment of an anastomotic bronchial dehiscence may be challenging. Mild partial dehiscence are best managed conservatively, with close bronchoscopic surveillance, antibiotic therapy and adequate bronchial debridement (Figure 3). A step forward in the management of dehiscence is the insertion of a stent. The stents are usually kept in place for 6 to 8 weeks after the healing has been observed. The use of self-expanding metallic stents in the management of a dehiscence are invariably associated to the development of granulation tissue that may require further debridation (27).
In complete dehiscence observed early after the transplant procedure, open surgical repair and flap bronchoplasty may be an option in selected patients. On occasion, a transplantectomy may be necessary. In our experience, patients with partial dehiscence were treated successfully with conservative measures and stent implantation.
Stenosis
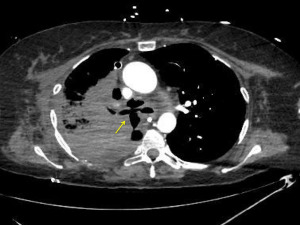
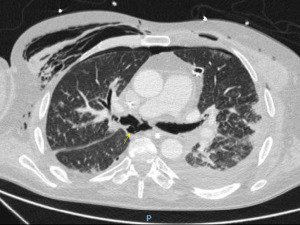
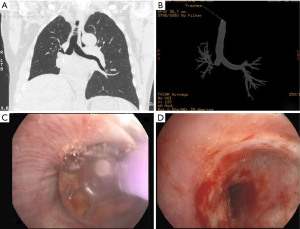
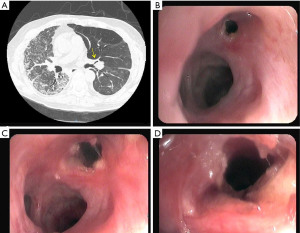
Bronchial stenosis is the most frequent airway complication after a lung transplant with an estimated incidence ranging from 5% to 30% (9,10) and is the natural consequence of previous dehiscence or infections and may appear within 2 to 9 months after transplantation. Mild stenoses are asymptomatic and are diagnosed in routine bronchoscopies. More severe stenoses present with various degrees of dyspnea, pneumonia episodes, or spirometric obstructive flow patterns (28). Stenoses may appear at the site of the anastomosis or distally (non-anastomosis stenoses) (Figure 4). A flexible bronchoscopy is the cornerstone for the diagnosis, but it does not provide accurate information regarding the length of the stenosis. In these cases an helical chest CT scan is needed to assess the complete picture of the stenosis, and to establish the most appropriate therapeutic approach (Figure 5).
The management of bronchial stenoses require a multimodal approach. Repeated endoscopic balloon bronchial dilatation procedures are usually the first step, especially useful in mild stenoses without granulation tissue, with improvement in symptoms and flow rates in up to 80% of patients (29) (Figure 6). Unfortunately, this procedure is insufficient to resolve moderate to severe stenoses. On occasion, combined procedures including debridation of necrotic tissue and electrocautery resection are required (Figures 7-9). In addition, in the absence of infection, the local injection of steroids and mitomycin-C to the stenotic area may reduce the incidence of restenosis (30).
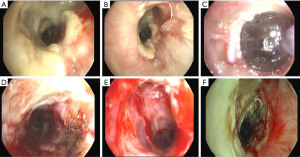
Severe and recurrent stenoses may require additional stent insertion. For this purpose, dilatation of the stenotic area facilitates the stent placement. Until recently, a silicon stent, was the first choice. However, they may migrate easily causing distal airway obstruction (31). Alternatively, covered metallic stents and, especially, self-expanding metallic stents (SEMS), and covered self-expanding metallic stents (Polyfex®) are usually the best choice to maintain the bronchial lumen with immediate relief of dyspnea (32,33). Some complications with the use of SEMS have been described: infection, granulation tissue formation, and migration, and difficulties with stent extraction (10,33).
Occasionally endoscopic procedures fail and surgery is required. Unfortunately, surgical options are very limited and with poor results. Bronchial anastomosis reconstruction, sleeve resections, or retransplantation have been documented (34). In our experience, these procedures are extremely unusual and their outcomes frustrating.
Granulation tissue
The growth of granulation tissue in the bronchial anastomosis is usually seen in up to 20% of patients within the first months after transplantation (10) and is frequently related to prior endoscopic procedures in the airway, such as the use of uncovered self-expanding metallic stents (33). Patients usually present with dyspnea, cough, hypoxia, or postobstructive pneumonia (35). Debridement is the modality of care but usually requires multiple procedures. For this purpose, the use of cryotherapy or Nd:YAG laser vaporization are equally useful (36,37). However, the use of cryotherapy is associated to less bronchial wall damage compared with the use of laser or electrocautery, but the effect is delayed several weeks (37). Argon plasma coagulation has also been used effectively in treating this granulation in the airway (38). Topical mitomycin-C may also be applied to reduce the proliferation of granulation tissue after debridement and stenting (30). Finally, high-dose-rate endobronchial brachytherapy has been reported to be useful in these patients with acceptable short-term improvement (35).
Malacia
Post-transplant bronchomalacia usually develops within 4 months after lung transplantation (4,5,9,10). Patients present wit dyspnea, stridor and inability to clear secretions. Malacic changes may be present at the site of bronchial anastomosis or, more commonly, affecting diffusely the airway distal to the anastomosis. The mechanism of bronchomalacia development, especially the diffuse form, is not well understood (4).
Flexible bronchoscopy is the primary diagnostic procedure. In addition, a dynamic chest CT scan may suggest the diagnosis (9,10). The therapeutic choices depend on the severity of functional impairment and airway narrowing. These include from conservative measures to non-invasive positive-pressure ventilation, and airway stenting. Given the dynamic nature of the malacic airway self-expanding metallic stent provide better palliation than silicone stents, being the first choice when stenting is required (9,10).
Other complications
Bronchopleural fistulas after lung transplantation are uncommon but carry a high morbidity and mortality. Patients present dyspnea, subcutaneous emphysema, pneumothorax, or a persistent air leak (39) (Figure 10). The cornerstone in the treatment of a bronchopleural fistula are the adequate drainage for local control of the infection followed by fistula closure and pleural space obliteration. Endoscopic application of methyl-2-cyanoacrylate, and fibrinogen plus thrombin may be useful, especially in high-risk patients (40). Also, the use of both covered metallic stents to occlude the fistula and endobronchial valves to manage persistent air leaks are useful for these patients (41) (Figure 11). Surgical options include open drainage, direct closure with flap reinforcement, trans-sternal bronchial closure, or thoracoplasty.
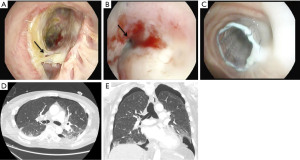
Infectious complications are common after lung transplantation. The exposition of the airway to the external environment in an immunosuppressed patient with an additional impairment of mucociliary clearance and disruption of lymphatic drainage are factors contributing to the infection in the airways, most commonly involving opportunistic pathogens. In addition, other bronchial complications, such as dehiscence, necrosis or repeated endobronchial procedures and stent implantations, make the airway at higher risk of infections.
Bronchial anastomotic infections are diagnosed by bronchoscopy. Pseudomonas and Staphylococcus aureus are the most frequent bacterial infections and specific systemic and aerosolized antibiotic therapy and local debridement are the basis of treatment (4,10).
Fungal infections, especially Aspergillus, are frequent in an ischemic anastomosis with a reported incidence as high as 24% (19), and may cause diffuse tracheobronchitis or infection at the anastomotic site. Frequent bronchoscopies, aggressive early empirical therapy and appropriate antifungal prophylaxis are the basic therapeutic approaches.
Vascular complications
Vascular complications after lung transplantation are infrequent with a reported overall incidence ranging from 2% to 15%, but associated to high morbidity and mortality (42,43). These complications comprise either the arterial or the venous anastomosis, being stenoses of these anastomoses the most common vascular complications.
The incidence of anastomotic pulmonary artery stenosis is less than 2%, and it is considered when the anastomotic diameter is less than 75% compared to the neighbouring vessels. Mild stenoses are commonly seen secondary to donor-recipient size discrepancies, but these are not associated to hemodynamic impairment (44).
Anastomotic venous stenoses are also rare, with a reported incidence around 15% (45) usually appearing early after completion of the transplant, and becoming the source of venous thrombosis and transplant failure. The inferior pulmonary veins are the most commonly involved due to their anatomical location.
To understand the causes of anastomotic vascular complications, we should take in mind the surgical technique for performing these anastomoses. In the standard lung transplant technique, vascular anastomoses are performed using a continuous suture technique. For this purpose, the recipient pulmonary artery and left atrial cuff are clamped. The pulmonary artery is divided on both the recipient and donor in an extent to avoid excessive lengh and kinking. The pulmonary venous anastomosis utilizes a standard left atrial cuff technique to create a wider venous confluent and to facilitate the appropriate orientation of the vascular edges to be anastomosed, to reduce the formation of thrombi (44).
Some concerns have been raised with vascular injuries related to the application of clamps when performing these anastomoses. The feasibility and safety of a “no clamp” technique has been described for vascular anastomoses in lung transplant (46). Potential advantages of the no clamp technique might include the reduction of potential damage to the vascular ends to be anastomosed. However, this practice is not routinely performed due to the necessity to perform the transplant on cardiopulmonary bypass.
Classification
Vascular anastomotic complications after lung transplantation have been classified into four types (42). Type 1 includes those anastomoses presenting kinking due to excessive length of the donor and recipient vascular edges, or distortion due to misalignment. Type 2 includes stenoses secondary to transposition of the donor vessel with respect to the recipient. Type 3 is a stenosis secondary to excessive anastomotic tightness. Type 4 includes complications related to the presence of intraluminal obstruction secondary to thrombosis. Type 5 refers to anastomotic stenoses due to extrinsic compression, frequently associated to the use of omental flaps.
Clinical presentation and diagnosis
The clinical features and imaging findings of the vascular anastomotic complications are often non-specific, and these should be suspected when symptoms have not improved despite being treated for the most common problems arising early after the lung transplant (primary graft dysfunction, infection, rejection). Common symptoms and signs are dry cough, dyspnea or ventilator dependence with unexplained hypoxia, pulmonary hypertension, and haemodynamic instability early after the transplant procedure. In addition, venous stenoses present pulmonary edema due to venous congestion (42,43).
In patients receiving a double lung transplant, the development of a unilateral vascular stenosis may be undetected due to the compensation by the contralateral graft. This might be the reason why vascular complications are more often diagnosed in single lung transplants (43).
A careful assessment of the vascular anastomoses should be done on completion of the transplant. This is of paramount importance to anticipate further graft problems and to allow an immediate surgical correction of the anastomosis. This is easily done by direct inspection and by the use of transesophageal echocardiography (TEE). This diagnostic modality requires a high level of expertise especially for the assessment of the venous anastomosis (47,48).
Some authors have reported the use of an intraoperative needle manometry line as an easy method to measure directly the pressure gradient across the anastomoses, to allow immediate surgical correction when required (43).
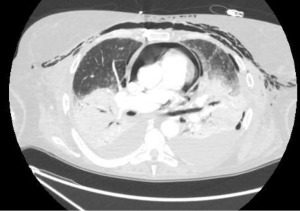
Postoperatively, a contrast enhanced chest CT scan is the investigation of choice to assess both the pulmonary artery and the venous anastomosis, which allows postprocessing techniques (49). It is readily obtained and interpreted and defines the extent and degree of the stenosis with less invasiveness than a pulmonary angiography. Additionally, the chest CT scan also provides information about the lung parenchyma and pleural space, allowing an appropriate differential diagnosis with other common causes of hypoxemia.
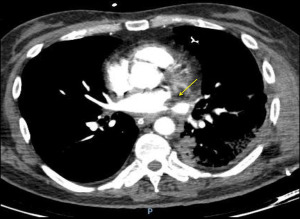
On contrast enhanced chest CT scan, vascular anastomoses are visualized as minimal folds on the walls of the blood vessels without a significant reduction in the diameter (49,50) (Figure 12). On the contrary, a significant reduction in the anastomotic vascular lumen may be seen in severe stenoses (Figure 13). In cases of pulmonary artery embolism, intraluminal filling defects are typical findings con chest CT scan.
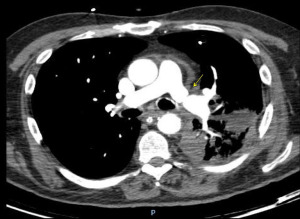
Pulmonary angiography is not routinely performed for diagnosis of vascular anastomotic problems, being reserved for cases in which a catheter-based intervention is planned. It can demonstrate filling defects and changes in vascular calibre. Additionally, trans-anastomotic pressure measurements might be useful, especially when the angiographic image is unclear. The assessment of the venous anastomosis requires delayed imaging to result in opacification of the pulmonary veins (Figure 14).
Other diagnostic tools include ventilation/perfusion scan and magnetic resonance angiography. The first may show absent perfusion secondary to pulmonary arterial occlusion but provides more limited information than contrast enhanced chest CT scan. The second is an important alternative to the chest CT scan in younger patients where radiation should be avoided (51).
Treatment
The management of a vascular complication after lung transplantation include from conservative measures to catheter-based interventions and surgical procedures. Although there are no clear recommendations to best manage these complications, the presence of a significant anatomic problem accompanied by graft dysfunction, requires urgent intervention. The type of intervention depends on the time that has elapsed from the completion of the transplant, the anatomical features of the vascular complication, the clinical status of the recipient, and the expertise of the transplant team.
In the immediate postoperative period, mild stenoses may be managed conservatively. In cases of anastomotic thrombosis, the initial treatment should consist of anticoagulation. However, in the coexistence of graft infarction a surgical intervention may be required, though it carries high operative mortality (42,43). If a pulmonary artery stenosis is detected intraoperatively, it should be corrected immediately, but those diagnosed several weeks after the transplant are best treated with endovascular procedures (52). In this situation, those surgical techniques aimed at preserving the lung graft are associated to high operative mortality rates. The required vascular clamping for surgical revision is associated to major complications and death (53). Alternatively, pulmonary artery stenoses are best managed with stent placement due to the elasticity of the lesions.
For those anastomotic complications arising within 2 weeks post-transplant, the choice of either a surgical or a catheter-based treatment are unclear. In general, a surgical approach is the best choice, as the integrity of the anastomosis is still friable given the short interval time from the time of surgery. The transplanted lung is cooled on cardiopulmonary bypass and cold pulmoplegic solution is utilized to prevent warm ischemia and infarction. Alternatively, some centers have reported successful stent placement in this postoperative period (54).
For vascular complications diagnosed late after the lung transplant, endovascular techniques are the best option for treatment. Percutaneous angioplasty with or without vascular endoprosthesis is usually successful, with low mortality and morbidity rates, for severe anastomotic stenosis of the pulmonary artery (55). In patients with complete venous obstruction, angioplasty with dilatation and endovascular stent placement may be useful (56), but, on occasion, re-transplantation or lobectomy may be required (57).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, Current Challenges in Thoracic Surgery for the series “Lung Transplantation”. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-22-2/coif). The series “Lung Transplantation” was commissioned by the editorial office without any funding or sponsorship. AA served as the unpaid Guest Editor of the series, and serves as an unpaid editorial board member of Current Challenges in Thoracic Surgery from November 2019 to October 2021. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wildevuur CR, Benfield JR. A review of 23 human lung transplantations by 20 surgeons. Ann Thorac Surg 1970;9:489-515. [Crossref] [PubMed]
- Yserbyt J, Dooms C, Vos R, et al. Anastomotic airway complications after lung transplantation: risk factors, treatment modalities and outcome-a single-centre experience. Eur J Cardiothorac Surg 2016;49:e1-8. [Crossref] [PubMed]
- FitzSullivan E, Gries CJ, Phelan P, et al. Reduction in airway complications after lung transplantation with novel anastomotic technique. Ann Thorac Surg 2011;92:309-15. [Crossref] [PubMed]
- Murthy SC, Blackstone EH, Gildea TR, et al. Impact of anastomotic airway complications after lung transplantation. Ann Thorac Surg 2007;84:401-9, 409.e1-4.
- Moreno P, Alvarez A, Algar FJ, et al. Incidence, management and clinical outcomes of patients with airway complications following lung transplantation. Eur J Cardiothorac Surg 2008;34:1198-205. [Crossref] [PubMed]
- Couraud L, Nashef SA, Nicolini P, et al. Classification of airway anastomotic healing. Eur J Cardiothorac Surg 1992;6:496-7. [Crossref] [PubMed]
- Shennib H, Massard G. Airway complications in lung transplantation. Ann Thorac Surg 1994;57:506-11. [Crossref] [PubMed]
- Dutau H, Vandemoortele T, Laroumagne S, et al. A new endoscopic standardized grading system for macroscopic central airway complications following lung transplantation: the MDS classification. Eur J Cardiothorac Surg 2014;45:e33-8. [Crossref] [PubMed]
- Crespo MM, McCarthy DP, Hopkins PM, et al. ISHLT Consensus Statement on adult and pediatric airway complications after lung transplantation: Definitions, grading system, and therapeutics. J Heart Lung Transplant 2018;37:548-63. [Crossref] [PubMed]
- Santacruz JF, Mehta AC. Airway complications and management after lung transplantation: ischemia, dehiscence, and stenosis. Proc Am Thorac Soc 2009;6:79-93. [Crossref] [PubMed]
- Alvarez A, Algar J, Santos F, et al. Airway complications after lung transplantation: a review of 151 anastomoses. Eur J Cardiothorac Surg 2001;19:381-7. [Crossref] [PubMed]
- Van De Wauwer C, Van Raemdonck D, Verleden GM, et al. Risk factors for airway complications within the first year after lung transplantation. Eur J Cardiothorac Surg 2007;31:703-10. [Crossref] [PubMed]
- Mason DP, Brown CR, Murthy SC, et al. Growing single-center experience with lung transplantation using donation after cardiac death. Ann Thorac Surg 2012;94:406-11; discussion 411-2. [Crossref] [PubMed]
- Ruttmann E, Ulmer H, Marchese M, et al. Evaluation of factors damaging the bronchial wall in lung transplantation. J Heart Lung Transplant 2005;24:275-81. [Crossref] [PubMed]
- Keshavjee SH, Yamazaki F, Yokomise H, et al. The role of dextran 40 and potassium in extended hypothermic lung preservation for transplantation. J Thorac Cardiovasc Surg 1992;103:314-25.
- Alvarez A, Salvatierra A, Lama R, et al. Preservation with a retrograde second flushing of Eurocollins in clinical lung transplantation. Transplant Proc 1999;31:1088-90. [Crossref] [PubMed]
- Grimm JC, Valero V 3rd, Kilic A, et al. Association Between Prolonged Graft Ischemia and Primary Graft Failure or Survival Following Lung Transplantation. JAMA Surg 2015;150:547-53. [Crossref] [PubMed]
- Yokomise H, Cardoso PF, Kato H, et al. The effect of pulmonary arterial flow and positive end-expiratory pressure on retrograde bronchial mucosal blood flow. J Thorac Cardiovasc Surg 1991;101:201-8.
- Nunley DR, Gal AA, Vega JD, et al. Saprophytic fungal infections and complications involving the bronchial anastomosis following human lung transplantation. Chest 2002;122:1185-91. [Crossref] [PubMed]
- Groetzner J, Kur F, Spelsberg F, et al. Airway anastomosis complications in de novo lung transplantation with sirolimus-based immunosuppression. J Heart Lung Transplant 2004;23:632-8. [Crossref] [PubMed]
- McAnally KJ, Valentine VG, LaPlace SG, et al. Effect of pre-transplantation prednisone on survival after lung transplantation. J Heart Lung Transplant 2006;25:67-74. [Crossref] [PubMed]
- Ramirez JC, Patterson GA, Winton TL, et al. Bilateral lung transplantation for cystic fibrosis. J Thorac Cardiovasc Surg 1992;103:287-94.
- Garfein ES, Ginsberg ME, Gorenstein L, et al. Superiority of end-to-end versus telescoped bronchial anastomosis in single lung transplantation for pulmonary emphysema. J Thorac Cardiovasc Surg 2001;121:149-54. [Crossref] [PubMed]
- Aigner C, Jaksch P, Seebacher G, et al. Single running suture--the new standard technique for bronchial anastomoses in lung transplantation. Eur J Cardiothorac Surg 2003;23:488-93. [Crossref] [PubMed]
- Pettersson G, Nørgaard MA, Arendrup H, et al. Direct bronchial artery revascularization and en bloc double lung transplantation--surgical techniques and early outcome. J Heart Lung Transplant 1997;16:320-33.
- Krishnam MS, Suh RD, Tomasian A, et al. Postoperative complications of lung transplantation: radiologic findings along a time continuum. Radiographics 2007;27:957-74. [Crossref] [PubMed]
- Mughal MM, Gildea TR, Murthy S, et al. Short-term deployment of self-expanding metallic stents facilitates healing of bronchial dehiscence. Am J Respir Crit Care Med 2005;172:768-71. [Crossref] [PubMed]
- Anzueto A, Levine SM, Tillis WP, et al. Use of the flow-volume loop in the diagnosis of bronchial stenosis after single lung transplantation. Chest 1994;105:934-6. [Crossref] [PubMed]
- De Gracia J, Culebras M, Alvarez A, et al. Bronchoscopic balloon dilatation in the management of bronchial stenosis following lung transplantation. Respir Med 2007;101:27-33. [Crossref] [PubMed]
- Ubell ML, Ettema SL, Toohill RJ, et al. Mitomycin-c application in airway stenosis surgery: analysis of safety and costs. Otolaryngol Head Neck Surg 2006;134:403-6. [Crossref] [PubMed]
- Murgu SD, Colt HG. Complications of silicone stent insertion in patients with expiratory central airway collapse. Ann Thorac Surg 2007;84:1870-7. [Crossref] [PubMed]
- Chhajed PN, Malouf MA, Tamm M, et al. Ultraflex stents for the management of airway complications in lung transplant recipients. Respirology 2003;8:59-64. [Crossref] [PubMed]
- Saad CP, Ghamande SA, Minai OA, et al. The role of self-expandable metallic stents for the treatment of airway complications after lung transplantation. Transplantation 2003;75:1532-8. [Crossref] [PubMed]
- Marulli G, Loy M, Rizzardi G, et al. Surgical treatment of posttransplant bronchial stenoses: case reports. Transplant Proc 2007;39:1973-5. [Crossref] [PubMed]
- Tendulkar RD, Fleming PA, Reddy CA, et al. High-dose-rate endobronchial brachytherapy for recurrent airway obstruction from hyperplastic granulation tissue. Int J Radiat Oncol Biol Phys 2008;70:701-6. [Crossref] [PubMed]
- Madden BP, Kumar P, Sayer R, et al. Successful resection of obstructing airway granulation tissue following lung transplantation using endobronchial laser (Nd:YAG) therapy. Eur J Cardiothorac Surg 1997;12:480-5. [Crossref] [PubMed]
- Maiwand MO, Zehr KJ, Dyke CM, et al. The role of cryotherapy for airway complications after lung and heart-lung transplantation. Eur J Cardiothorac Surg 1997;12:549-54. [Crossref] [PubMed]
- Keller CA, Hinerman R, Singh A, et al. The use of endoscopic argon plasma coagulation in airway complications after solid organ transplantation. Chest 2001;119:1968-75. [Crossref] [PubMed]
- Lois M, Noppen M. Bronchopleural fistulas: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65. [Crossref] [PubMed]
- Chang CC, Hsu HH, Kuo SW, et al. Bronchoscopic gluing for post-lung-transplant bronchopleural fistula. Eur J Cardiothorac Surg 2007;31:328-30. [Crossref] [PubMed]
- Toma TP, Kon OM, Oldfield W, et al. Reduction of persistent air leak with endoscopic valve implants. Thorax 2007;62:830-33. [Crossref] [PubMed]
- Batra K, Chamarthy MR, Reddick M, et al. Diagnosis and interventions of vascular complications in lung transplant. Cardiovasc Diagn Ther 2018;8:378-86. [Crossref] [PubMed]
- Siddique A, Bose AK, Özalp F, et al. Vascular anastomotic complications in lung transplantation: a single institution's experience. Interact Cardiovasc Thorac Surg 2013;17:625-31. [Crossref] [PubMed]
- Yokoyama Y, Chen-Yoshikawa TF, Nakajima D, et al. Various techniques for anastomosis of pulmonary arteries with size mismatch during lung transplantation. JTCVS Tech 2021;9:192-4. [Crossref] [PubMed]
- Schulman LL, Anandarangam T, Leibowitz DW, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr 2001;14:806-12. [Crossref] [PubMed]
- Mohite PN, Garcia-Saez D, Sabashnikov A, et al. No-clamp technique for pulmonary artery and venous anastomoses in lung transplantation. J Heart Lung Transplant 2014;33:1133-8. [Crossref] [PubMed]
- Michel-Cherqui M, Brusset A, Liu N, et al. Intraoperative transesophageal echocardiographic assessment of vascular anastomoses in lung transplantation. A report on 18 cases. Chest 1997;111:1229-35. [Crossref] [PubMed]
- Felten ML, Michel-Cherqui M, Sage E, et al. Transesophageal and contact ultrasound echographic assessments of pulmonary vessels in bilateral lung transplantation. Ann Thorac Surg 2012;93:1094-100. [Crossref] [PubMed]
- DeFreitas MR, McAdams HP, Azfar Ali H, et al. Complications of Lung Transplantation: Update on Imaging Manifestations and Management. Radiol Cardiothorac Imaging 2021;3:e190252. [Crossref] [PubMed]
- Hemmert C, Ohana M, Jeung MY, et al. Imaging of lung transplant complications. Diagn Interv Imaging 2014;95:399-409. [Crossref] [PubMed]
- Ingrisch M, Maxien D, Meinel FG, et al. Detection of pulmonary embolism with free-breathing dynamic contrast-enhanced MRI. J Magn Reson Imaging 2016;43:887-93. [Crossref] [PubMed]
- Najafizadeh K, Daneshvar A, Dezfouli AA, et al. Pulmonary artery stenosis shortly after lung transplantation: successful balloon dilation and stent insertion in one case. Ann Transplant 2009;14:52-5.
- Anaya-Ayala JE, Loebe M, Davies MG. Endovascular management of early lung transplant-related anastomotic pulmonary artery stenosis. J Vasc Interv Radiol 2015;26:878-82. [Crossref] [PubMed]
- Chen F, Tazaki J, Shibata T, et al. Stent angioplasty for a kink in the pulmonary artery anastomosis soon after living-donor lobar lung transplantation. Ann Thorac Surg 2011;92:e105-6. [Crossref] [PubMed]
- Grubstein A, Atar E, Litvin S, et al. Angioplasty using covered stents in five patients with symptomatic pulmonary artery stenosis after single-lung transplantation. Cardiovasc Intervent Radiol 2014;37:686-90. [Crossref] [PubMed]
- Jobanputra YB, Kapadia SR, Johnston DR, et al. Pulmonary Vein Stenosis Following Single-Lung Transplantation Successfully Treated with Intravascular Ultrasound-Guided Angioplasty and Stent Placement. Am J Case Rep 2017;18:1289-95. [Crossref] [PubMed]
- Jing L, Chen W, Zhai Z, et al. Pulmonary vein stenosis after lung transplantation: a case report and literature review. Ann Transl Med 2021;9:181. [Crossref] [PubMed]
Cite this article as: Alvarez A. Anastomotic complications after lung transplantation. Curr Chall Thorac Surg 2023;5:10.