Lung donation after circulatory death
Introduction
Donation after circulatory death (DCD) defines organs procurement from a donor whose death has been determined using circulatory criteria, as opposed to a declaration based on neurological criteria. Death is ultimately assumed as the loss of brain function due to the prolonged absence of encephalic perfusion. In this situation, the primum movens is an irreversible cardiac arrest with an adequate period of asystole. These criteria are established in order to act in accordance with the dead donor rule, as it happens for the donation after brain death (DBD) setting. The donor must already be dead before any vital organ is removed, and organ harvesting must not cause the donor’s death. The very first lung transplantation was performed with organs procured from a DCD donor (1). Only after the Harvard criteria were published, both the brain death concept and the DBD path gained worldwide traction, in light of the encouraging clinical results (2). Interest in DCD was renewed in the last 20 years thanks to Love’s work and to Steen’s experiences in 2001 (3,4). Nowadays, DCD donors represent a valuable resource to contrast the long-standing problems of lung donor shortage and waiting list mortality. Moreover, the use of grafts from DCDs helps to avoid the pro-inflammatory cytokines and the catecholamines ‘storms’ that occur after brain death, which pose a significant threat to the lung rather than to the other organs. On the other hand, lungs from DCDs are exposed to warm ischemia and its potential damages.
Pre-clinical experiences
The resistance of pulmonary cells to ischemia is well known as well as the possibility for them to dissociate ischemia from hypoxia by using the oxygen supplied from ventilation. In 1991, Egan published a study on the ability of lung cells to endure ischemia for an adequate period of time using a single left lung transplant canine model, proving the feasibility of procurement from non-heart-beating donors (5). Lung harvesting was performed 1, 2 and 4 hours after the donor’s death, and grafts were stored on ice for 4 hours before carrying out transplantation. The Authors claimed that lung retrieval from DCD donors was safe and effective. A few years later, studies on pulmonary cell injury after cardiocirculatory arrest demonstrated that cell death was delayed by supplying oxygen via mechanical ventilation (6). The work of Van Raemdonck et al. further confirmed the key role of ventilation in this scenario. Ventilation-related parameters, pulmonary vascular resistance and pulmonary oedema were compared using a DCD rabbit model after delayed pulmonary flushing. The lungs received six different post-mortem protocols: immediate flushing (control DBD group), lung deflation (control DCD group), inflation with room air, inflation with pure oxygen (fraction of inspired oxygen, FiO2=100%), ventilation with room air, ventilation with 100% nitrogen or ventilation with 100% oxygen. Lungs in each group were flushed after different ischemic times to study the organ tolerance to ischemia. The Authors concluded that delayed lung flushing in deflated lungs could result in pulmonary oedema, and that prevention of alveolar collapse via post-mortem pulmonary inflation protected the lungs from suffering warm ischemia time (WIT) injuries (7). To investigate the effects of donor prone positioning on lung preservation in DCD, the Toronto group used a controlled DCD swine model. After death determination, pigs remained supine or were positioned prone, ventilation was resumed for 5 minutes and the endotracheal tube was clamped after recruitment manoeuvres. After 3 hours of WIT, 6 hours of cold preservation and 6 hours of normothermic machine perfusion, the results show a better tolerance to WIT, less atelectasis and apoptosis in the prone positioned group. These results confirmed once again the importance of ventilation in lung preservation (8). Great interest has always been given to the intrapulmonary thrombi formation after lung procurement from DCD and subsequent reperfusion injury. The group from Leuven adopted four different approaches in a porcine DCD model: in one group, they administrated heparin post-mortem, for the second group in situ retrograde flushing with Perfadex solution was employed, for the third one they adopted a combined approach (heparinization and retrograde flushing) and the last one received no intervention (control group) (9). The lungs were procured and then assessed in an ex-vivo model and ventilation and vascular resistance parameters were analysed. The retrograde flushing and the combined approach group showed better results in terms of both types of variables. The Lund group designed an experimental study on pigs to investigate the role of alteplase in addition to Perfadex during lung flushing in the prevention of lung thrombosis (10). Grafts were then assessed via ex-vivo lung perfusion (EVLP). Lung functionality was excellent in all 12 cases; however, despite slightly better blood gases and pulmonary vascular resistance in the alteplase group, the difference between the two groups was not statistically significant.
Classification
The term “DCD” encompasses clinical situations of great variety, hence the necessity to establish a classification of these donors. The most known and widely used is the Maastricht classification, which was drafted in 1995 at the First International Workshop on DCD (Table 1) (11). This classification identifies two main classes: uncontrolled DCD (uDCD) includes categories I and II, where the cardiac arrest is unanticipated and therefore the donor’s medical history is unknown. Category I donors are those found dead after an unexpected cardiac arrest; no cardiopulmonary resuscitation manoeuvres are attempted by the medical team. For category II donors, the cardiac arrest is witnessed, and cardiopulmonary resuscitation is unsuccessful. Instead, the third and fourth category are defined as controlled donors (cDCD): the donor’s medical history is known, and both donors and graft functions are evaluated in time, before withdrawal of life-sustaining therapy (WLST). In the case of a category III donor, graft harvesting takes place after an expected cardiac arrest, and therefore it can be planned in advance. For category IV donors, a sudden cardiac arrest occurs after brain-death determination during donor life-management. Numerous other classifications have been proposed, presenting more detailed sub-classifications which specifically address the cardiac arrest location (in- or out-of hospital). However, the various classifications share the same basic layout. Lastly, since some countries allow medically assisted death (euthanasia), this type of donors was included in the classification as category V (12). These classifications are not only semantics, but rather necessary tools to correctly share and compare the experiences of centres around the world. It is important to point out that each setting has its own specificity which reflects the challenges brought by the DCD program itself.
Table 1
| Category | Definition |
|---|---|
| Uncontrolled I | Death on arrival |
| Uncontrolled II | Unsuccessful resuscitation |
| Controlled III | Awaiting cardiac arrest |
| Controlled IV | Cardiac arrest while brain dead |
DCD, donation after circulatory death.
Differences around the world
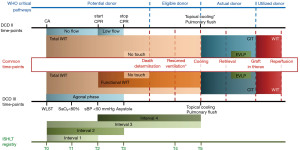
Despite the efforts made to uniform pathways and definitions, the debate about DCD remains intense. DCD is met around the world with extremely diverse approaches, going so far as to the complete prohibition of DCD donor’s usage in some countries as, for example, in Germany. This variety is mostly related to the influence of local factors such as culture, logistics, law, ethics, and religion. Furthermore, every organ has its own necessities due to different ischemic tolerance, requiring dedicated choices in terms of preservation and procurement techniques. The net result is the development of a range of DCD programs, with different approaches, in a controlled or uncontrolled setting, addressed to single or multiorgan procurement. The main differences among protocols are related to the required length of no-touch period and the definition of ante- versus post-mortem interventions permitted. In fact, while in most countries only 5 minutes of asystole are needed for death declaration, 10 minutes are mandatory in Switzerland, Austria, and the Czech Republic. Italy is in a unique situation, being the only country requiring 20 minutes of no touch period (Table 2) (13). Most countries allow ante-mortem interventions in order to promote organ preservation, as long as they do not accelerate death and do not cause discomfort to the patient. Nevertheless, some legislations prohibit any kind of manoeuvres before the declaration of death (i.e., UK). Consent, as well as the usefulness of drugs administration before circulatory arrest, are still debated; data from the International Society of Heart and Lung Transplantation (ISHLT) DCD registry show that the most used premortem practices include heparin (53% of DCD donors), corticosteroids (58%) and fibrinolytics (0.2%) (14). However, some centres do not employ heparinization before declaration of death, and there is no clear evidence regarding its influence on the outcome of lung transplanted patients (15). Obviously, any drug administered post-mortem requires chest compression to ensure it circulated. Nevertheless, patients who are potential donors are not to be considered donors until death occurs, and they must be treated accordingly, without accelerating the death of the patient. This fundamental principle of non-malfeasance must be followed, while the need to observe the patient’s will to donate must also be respected. Physicians must also take into account how important it is not to waste the chance of retrieving a potentially suitable organ. Balancing these elements out by taking all these ethical issues is an extremely delicate process. The wide variety of definitions and terms currently in use in discussing DCD makes it difficult to evaluate and compare the reported experiences on the practice, especially when discussing the extent of the lung ischemic injury. Lung damage during grafts preservation and procurement phases is temperature-dependent; we identify a warm and a cold ischemia time (WIT and CIT, respectively) over the process. Ischemia time identifies the time span when organ perfusion is reduced or absent. Differently from WIT, CIT occurs when the organ cooling starts, both by topical and/or pulmonary flushing. Notably, whenever the organs are perfused with a low flow, as during the agonal phase, until the systolic blood pressure lowers under 50 mmHg, it is defined as a functional WIT. The WIT effects are considered more relevant to organ function and the limits of warm ischemia tolerance are being extensively investigated, even though this concept is not always accurately reported, described or calculable (16). Although WIT is often defined as the period going from cardiac arrest to cold flush, we need to consider that the resumption of ventilation may play a relevant role in the lung preservation procedure too (17). In an experimental porcine model, Miyoshi and colleagues found that while lung hypoxia before cardiac arrest had a detrimental effect on pulmonary function, hypotension did not in turn affect the outcome (18). They suggested that the cessation of ventilation is a potential point in time for the onset of WIT. Levvey too proposed different definitions of WIT including pivotal points in the procurement timeline; one of them overlapped with the time period between WLST and the resumption of ventilation (16). On the other hand, the CIT period usually begins at the moment of the topical cooling, which often, but not always, coincides with cold pulmonary flushing. In the protocol adopted by the Spanish group, for example, topical cooling is used to preserve the organs in situ before the beginning of the harvesting procedure (19). Lastly, the use of machine perfusion techniques in DCD affects the ischemic time, as it maintains organs ventilated and perfused within a normothermic temperature range. In Figure 1, we report an illustration of the clinical pathways and timelines that describe such a complex process. In particular, we attempted to merge the two timelines of controlled and uncontrolled setting, pointing out the common phases. We also enclose the changing status of the patients over the donation process as defined by the World Health Organization to reaffirm the requirement that organ retrieval must not result in the death of the patient.
Table 2
| No touch period (minutes) | Country |
|---|---|
| 5 | Belgium, France, The Netherlands, Spain, United Kingdom, USA |
| 10 | Austria, Czech Republic, Switzerland |
| 15 | Latvia |
| 20 | Italy |
DCD, donation after circulatory death.
Controlled DCD: category III donors
The controlled setting represents most of the DCD donors: 94.1% of the DCD lung transplant reported in the ISHLT DCD registry were cDCDs (14). This clinical scenario, however, is not free from difficulties, particularly from an ethical point of view. In fact, the issue of therapies withdrawal in the intensive care units (ICU) is far from simple. The controlled setting allows physicians to assess the grafts before WLST and the organization of procurement teams can be properly planned. The management of potential donors in the ICU is of pivotal importance, as well as in the DBD path. In most centres, donors are extubated, but there is no consensus regarding this approach; it might act as a protection from aspiration and gasping damages but, on the other hand, it may prolong the agonal period by preventing the upper airways from collapsing (21). The nasogastric tube is used for suction to prevent inhalation later during extubation; enteral nutrition should always be suspended a few hours before WLST.
Ischemic time in cDCD
Treatment withdrawal can take place in the ICU or in the operating room; the latter is often associated with shorter acirculatory phase (from asystole to cold flushing). We previously mentioned that the correct definition of WIT presents difficulties, as during the first part of the agonal phase (from WLST to asystole) organs are perfused, yet the pressure of perfusion is not always adequately effective. In order to better define WIT in a controlled DCD setting, time intervals were then reported by the ISHLT DCD-registry (20) (Figure 1). Precise limits of the acceptable agonic time are also established. The Groningen group limits the agonic time to 60 minutes, while Australian protocols range from 60 to 90 minutes. British protocols extend the tolerability up to 2 hours following the WLST or up to 1 hour following the onset of WIT. The ISHLT DCD registry reported a median time interval of 15 minutes from WLST to cardiac arrest (Interval 2) and of 32 minutes from WLST to cold flush (Interval 3) (Figure 1) (14). Great interest for the possibility of predicting potential determinants of the agonic time length has been shown over the years; a multicentre study on 191 potential DCD donors found younger age, higher FiO2 and mode of ventilation to be independently associated with a shorter time to death (22). The clinically acceptable length of warm ischemia, however, it is yet to be determined. A recent retrospective study by Levvey et al. aimed to study the impact of both agonal and WIT on early survival after lung transplantation with organs from cDCD donors. Their analyses, which were based on data from the ISHLT DCD registry, did not show any association between an agonal phase or WIT up to 60 minutes and a worsening in early survival (23). Recently, we have published the results of our first five cDCD donors: we transplanted lungs with a maximum total WIT (Interval 3, from WLST to cold flush) of 284 minutes and a maximum functional WIT (Interval 4, from the drop of the systolic pressure below 50 mmHg to lung cold flush) of 261 minutes, with favourable outcomes despite the extended WIT (17).
Assessment, preservation techniques and procurement protocols in cDCD
In DCD clinical paths it is possible to systematically identify two pivotal moments for the preservation and assessment of the lung. The first, in situ, occurs in the donor’s body while the second, ex situ, follows the organ retrieval. In the controlled setting, the past and recent medical history of the donor is known, the intensivists can perform the usual tests for assessing the quality of the lungs, and the procurement surgeon can evaluate them directly by bronchoscopy and after sternotomy. After harvesting, the evaluation can occur at the back table or, in case of doubt about extended criteria donors or long ischemic times, by machine perfusion. Regarding the preservation, after the declaration of death, organ retrieval is typically performed expeditiously. The majority of the procurement protocols include rapid sternotomy and pulmonary arterial cannulation; some Authors prefer topical cooling immediately after sternotomy, ensuring a rapid slow-down of cellular metabolism. This choice of approach has the undeniable advantage of minimizing the ischemia time from cardiac arrest and the need for preservation. However, due to its characteristics, the lung can endure longer ischemic times once ventilation is resumed. Rapid pulmonary artery cannulation and anterograde flush can imply blind perfusion of the lungs. The lack of management of pleural effusion and/or parenchymal atelectasis due to lung de-recruitment could result in inhomogeneous cold perfusion. Following classic experience with brain-dead donors’ procurement, avoiding those risks should be the mainstay of DCD protocols too, even at the expense of relatively longer ischaemia times for lungs (Figure 2). In contrast, an excessive WIT length due to more or less modifiable reasons is certainly to be countered for the other organs. The introduction of abdominal normothermic regional perfusion (NRP) allows for adequate reconditioning and evaluation of abdominal organs (Figure 3) (17,24). The subsequent phases follow those of usual DBD lung procurement; Perfadex (low potassium dextran solution) is the most commonly used preservation solution and is frequently supplemented with additional buffers and vasodilators. A generous retrograde flush from pulmonary veins is an important step in the management of grafts from DCD (9).
Lung transplantation outcomes after cDCD
Lung transplantation after cDCD shows adequate early clinical outcomes in terms of primary graft dysfunction (PGD) and acute rejection. In addition, there are no differences in 1-year survival rate compared to patients who received a graft from DBD (25). We have recently published a meta-analysis on lung transplantation from controlled donors: the results showed that the outcomes of grafts procured from cDCD are comparable to those from DBD in terms of 1-year survival, PGD and 1-year freedom from chronic lung allograft dysfunction (26). However, we found a higher risk of airway complications and poorer long-term survival in cDCD transplanted patients; this association, however, could be affected by the heterogeneity of the definition of airway complication and by recipients’ pre-existing conditions. In general, comparing experiences cannot ensure comprehensive results because of the non-homogeneity of recipients’ selection. Some centres do not use DCD grafts for the sickest listed patients. This probably reflects the overall impression that lungs from DCD are somehow inferior in quality to those obtained from DBD. On the other hand, more recent studies openly stated that recipient selection according to donor type has been generally avoided. Barbero et al. published a study comparing the outcomes of lung transplantation from cDCD vs. DBD carried out at Royal Papworth Hospital: they did not find statistically significant differences between the two groups in terms of early and mid-term outcomes; notably, the results showed that this group tended to use graft from DCDs for older recipients (15).
Uncontrolled DCD: category II donors
A donor is defined as uncontrolled when the circulatory arrest is sudden, unexpected, and irreversible. A witnessed cardiac arrest with subsequent unsuccessful cardiopulmonary resuscitation identifies Maastricht category II donors; the circulatory arrest can take place in or out-of-hospital. In this setting, the ethical aspect has a less significant impact than on controlled donors since there is no need for treatment suspension, but only for the observation of the ineffectiveness of resuscitation manoeuvres. Nevertheless, this type of donors represents a major challenge to physicians: the donor’s clinical history is unknown, a thorough organ evaluation is more complicated than in the controlled setting, and organizational and logistical aspects are difficult to deal with. A surely relevant aspect is the donation system structure of the country; the need to obtain relatives’ consent before being able to consider the patient as a donor, can represent an obstacle within an already difficult path. The organs must adequately be preserved inside the donor before the retrieval because time is necessary for organizing matters and to obtain consent. Some countries adopted an “opt in” system (i.e., citizens need to register and give their consent in order to be considered as potential donors); other states, such as Austria and Spain, preferred an “opt out” system, where the consent to donate is presumed unless the potential donor has declared otherwise. More specifically, the most commonly enforced option is the “soft” opting out law: the will of the potential donor is presumed in favour of donation in absence of a refusal of consent, but the donor’s family can declare their opposition and has the last say. The opting out system, where applied, obviously makes the organisational aspects of procurement from category II donors easier. While the experience with this particular clinical setting is now consolidated in Spain (27-29), reports from around the world are much more limited (30,31).
Ischemic time in uDCD
In the uncontrolled setting, WIT can be divided in two phases: a no-flow period, from the circulatory arrest to the beginning of cardiopulmonary resuscitation, and a low flow period, while resuscitation manoeuvres are maintained. Each group established a maximum tolerated period of WIT but most centres recommend a WIT <150 minutes and a total preservation time <240 minutes: namely, the Spanish protocol accepts lungs with a maximum of 120 minutes (this threshold was recently increased to 150 minutes) (21,28); the Toronto group set a maximum 3 hours period from death declaration to the beginning of procurement (31). In our centre, we generally accept a total WIT of 150 minutes maximum.
Assessment, preservation techniques and procurement protocols in uDCD
The uncontrolled setting presents specific difficulties concerning the various phases of the process. Donors’ medical history is usually unknown, and the ICU medical staff is not able to perform the necessary tests to thoroughly evaluate the functionality of the grafts. The in situ evaluation usually consists in a bronchoscopy, requiring particular attention to any sign of inhalation, and a direct inspection after sternotomy. Anyway, since the quality of the lungs cannot be comprehensively assessed, the ex situ evaluation after retrieval usually takes a pivotal role in the uncontrolled setting. Steen performed the first successful lung transplantation after EVLP evaluation from uDCD donor (4). He initially described procedures for rapid topical cooling and many centres have adopted this technique since then. The Madrid group then described their own uDCD protocol, which includes cardiopulmonary resuscitation manoeuvres until the declaration of death (19). After the “hands off” period, heparin is administrated, and the donor is connected to an ECMO system, while a Fogarty catheter is inserted through a femoral artery and inflated above the diaphragm for better preservation of the abdominal organs. Lungs are then topically cooled with four litres of Perfadex solution (4 ℃) via two chest tubes on each side; during this phase, ventilation is suspended. Since Spain has adopted a presumed consent law, these interventions can take place before obtaining the next of kin’s permission to proceed with procurement. Once consent is obtained, Perfadex is drained, ventilation is resumed, and lungs are harvested. After a long pre-clinical and educational phase, in 2014 our lung-DCD project took off, starting from isolated lung procurements from uncontrolled DCD donors (Maastricht class II). In our protocol, after clinical diagnosis of death, a recruitment manoeuvre is performed; during the required 20 minutes of “hands off” time, continuous positive airway pressure is maintained. Following declaration of death, consent from next of kin is obtained and then heparin is administrated, cardiopulmonary resuscitation is reinstated for three minutes and ventilation is resumed. Unlike the Spanish group, we adopted a normothermic open-lung procurement technique: until cold flushing, lungs are preserved only by protective mechanical ventilation, namely without chest tubes. After harvesting, we perform an ex-situ evaluation with EVLP run with a low-flow, open atrium and low haematocrit technique. The experience with this approach confirmed the feasibility of lung donation from uDCD and gave us the green light to transplant lungs even with extended WIT (30). The Toronto group also employs ventilation as a strategy for lung preservation; their recently published experience with uDCD confirms the safety and effectiveness of this approach (31). As organ function cannot be assessed before death or procurement, grafts usually require ex-situ evaluation prior to transplantation; machine perfusion techniques and their application in cases of DCD donors are briefly described below. Some of the Spanish groups recently started using EVLP, even though the technique is generally not favoured by the group. In fact, they often assess oxygenation capacity in situ after sternotomy by performing arterial blood gases after a single pulmonary flush of 300 mL of venous donor blood mixed with Perfadex (27,28).
Lung transplantation outcomes after uDCD
Uncontrolled DCDs are still an underused option for organ retrieval, probably due to the concerns for PGD, airway complication and chronic lung allograft dysfunction. Since only few centres have an uDCD program and experience around the world is still very limited, there are no meta-analysis on the outcomes of lung transplantation after uDCDs. A retrospective study published by the Madrid group aimed to compare the outcomes of lung transplantation from uDCD and from DBD donors. They found that PGD was significantly higher in the uDCD group only when comparing PGD grade 2 and 3 combined. This, however, was not associated with worse early-term outcomes, while overall survival was poorer in the uDCD group (29). Although the reported PGD and mortality rates are higher than expected after lung transplantation from DBD and cDCD donors, it appears that uDCD can provide viable, good quality organs. In a brief communication published in 2018, Suberviola et al. reported their experience with uDCDs, showing a 5-year survival of 87.5% (28). In our centre, we perform lung transplantation after uDCD, with good results both in terms of survival and quality of life. In particular, the first case recipient is alive and in good condition, showing no sign of chronic rejection almost six years after transplantation. The first report on a series of 5 lung transplantations from uDCD donors performed in North America demonstrated adequate early outcomes (31).
Machine perfusion technique in DCD
Machine perfusion represents a valuable resource for lung assessment in both controlled and uncontrolled settings. According to the ISHLT registry, DCD grafts assessment via EVLP was only employed in 15% cases of Maastricht III, and it was mostly used in one centre (14). In fact, in case of standard criteria cDCD donors, EVLP is not considered mandatory anymore, and the results of different experiences showed the safety of lung transplantation with this type of grafts without employing an ex-vivo evaluation technique (25,32). Most centres do not routinely use EVLP platform for lung evaluation after procurement, as a proper functional assessment is achievable right before the WLST. Nevertheless, EVLP remains an appropriate tool for the evaluation of graft function whenever extended criteria donors are considered (33,34). Moreover, the use of EVLP is advised in case of a prolonged interval between WLST and cardiac arrest (>60 minutes) or if pulmonary oedema, poor compliance or high-risk history are likely to arise (35). Only a few centres accept DCD donors with “extended criteria” such as smoking history of >20 pack/years, intensive care unit stay >5 days, PaO2/FiO2 <400 mmHg, and abnormal chest X-ray; in this case, it is advisable to perform a thorough assessment via machine perfusion. Most centres routinely use EVLP to assess the functionality of grafts procured in the uncontrolled setting (31,36). In this particular clinical scenario, machine perfusion is also a valuable tool to expand the times and to preserve the organs while dealing with the logistical issues. More recently, an integrated and transportable machine perfusion system, the Organ Care Support (OCS) Lung (Transmedics, Andover, MA, USA), has been used for lung assessment and reconditioning. The Harefield Hospital group presented the first experience with OCS in the DCD setting: they noted that this system allows for continuous ventilation, recruitment manoeuvres and further bronchoscopic evaluations (37).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Antonio Alvarez) for the series “Lung Transplantation” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-148/coif). The series “Lung Transplantation” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Hardy JD, Webb WR, Dalton ML Jr, et al. Lung homotransplantation in man. JAMA 1963;186:1065. [Crossref] [PubMed]
- A definition of irreversible coma. Report of the Ad Hoc Committee of the Harvard Medical School to Examine the Definition of Brain Death. JAMA 1968;205:337-40.
- D’Alessandro AM, Hoffmann RM, Knechtle SJ, et al. Controlled non-heart-beating donors (NHBDs): a potential source of extrarenal organs. Transplant Proc 1995;27:707-9.
- Steen S, Sjöberg T, Pierre L, et al. Transplantation of Lungs From a Non-Heart-Beating Donor. Lancet 2001;357:825-9. [Crossref] [PubMed]
- Egan TM, Lambert CJ Jr, Reddick R, et al. A strategy to increase the donor pool: use of cadaver lungs for transplantation. Ann Thorac Surg 1991;52:1113-20; discussion 1120-1. [Crossref] [PubMed]
- D'Armini AM, Roberts CS, Griffith PK, et al. When does the lung die? I. Histochemical evidence of pulmonary viability after "death". J Heart Lung Transplant 1994;13:741-7.
- Van Raemdonck DEM, Jannis NCP, Rega FRL, et al. Extended preservation of ischemic pulmonary graft by postmortem alveolar expansion. Ann Thorac Surg 1997;64:801-8. [Crossref] [PubMed]
- Watanabe Y, Galasso M, Watanabe T, et al. Donor prone positioning protects lungs from injury during warm ischemia. Am J Transplant 2019;19:2746-55. [Crossref] [PubMed]
- Van De Wauwer C, Neyrinck AP, Rega FR, et al. Retrograde flush is more protective than heparin in the uncontrolled donation after circulatory death lung donor. J Surg Res 2014;187:316-23. [Crossref] [PubMed]
- Liersch-Nordqvist A, Fakhro M, Pierre L, et al. The impact of alteplase on pulmonary graft function in donation after circulatory death - An experimental study. Ann Med Surg (Lond) 2017;22:1-6. [Crossref] [PubMed]
- Kootstra G, Daemen JH, Oomen AP. Categories of nonheart-beating donors. Transplant Proc 1995;27:2893-4.
- Thuong M, Ruiz A, Evrard P, et al. New classification of donation after circulatory death donors definitions and terminology. Transpl Int 2016;29:749-59. [Crossref] [PubMed]
- Domínguez-Gil B, Haase-Kromwijk B, Van Leiden H, et al. Current situation of donation after circulatory death in European countries. Transpl Int 2011;24:676-86. [Crossref] [PubMed]
- Van Raemdonck D, Keshavjee S, Levvey B, et al. Donation after circulatory death in lung transplantation-five-year follow-up from ISHLT Registry. J Heart Lung Transplant 2019;38:1235-45. [Crossref] [PubMed]
- Barbero C, Messer S, Ali A, et al. Lung donation after circulatory determined death: a single-centre experience. Eur J Cardiothorac Surg 2019;55:309-15. [Crossref] [PubMed]
- Levvey BJ, Westall GP, Kotsimbos T, et al. Definitions of warm ischemic time when using controlled donation after cardiac death lung donors. Transplantation 2008;86:1702-6. [Crossref] [PubMed]
- Palleschi A, Tosi D, Rosso L, et al. Successful preservation and transplant of warm ischaemic lungs from controlled donors after circulatory death by prolonged in situ ventilation during normothermic regional perfusion of abdominal organs. Interact Cardiovasc Thorac Surg 2019;29:699-705. [Crossref] [PubMed]
- Miyoshi K, Oto T, Otani S, et al. Effect of donor pre-mortem hypoxia and hypotension on graft function and start of warm ischemia in donation after cardiac death lung transplantation. J Heart Lung Transplant 2011;30:445-51. [Crossref] [PubMed]
- de Antonio DG, Marcos R, Laporta R, et al. Results of clinical lung transplant from uncontrolled non-heart-beating donors. J Heart Lung Transplant 2007;26:529-34. [Crossref] [PubMed]
- Cypel M, Levvey B, Van Raemdonck D, et al. International Society for Heart and Lung Transplantation Donation after Circulatory Death Registry Report. J Heart Lung Transplant 2015;34:1278-82. [Crossref] [PubMed]
- Erasmus ME, Van Raemdonck D, Akhtar MZ, et al. DCD lung donation: donor criteria, procedural criteria, pulmonary graft function validation, and preservation. Transpl Int 2016;29:790-7. [Crossref] [PubMed]
- Suntharalingam C, Sharples L, Dudley C, et al. Time to cardiac death after withdrawal of life-sustaining treatment in potential organ donors. Am J Transplant 2009;9:2157-65. [Crossref] [PubMed]
- Levvey B, Keshavjee S, Cypel M, et al. Influence of lung donor agonal and warm ischemic times on early mortality: Analyses from the ISHLT DCD Lung Transplant Registry. J Heart Lung Transplant 2019;38:26-34. [Crossref] [PubMed]
- Oniscu GC, Siddique A, Dark J. Dual temperature multi-organ recovery from a Maastricht category III donor after circulatory death. Am J Transplant 2014;14:2181-6. [Crossref] [PubMed]
- Costa J, Shah L, Robbins H, et al. Use of Lung Allografts From Donation After Cardiac Death Donors: A Single-Center Experience. Ann Thorac Surg 2018;105:271-8. [Crossref] [PubMed]
- Palleschi A, Rosso L, Musso V, et al. Lung transplantation from donation after controlled cardiocirculatory death. Systematic review and meta-analysis. Transplant Rev (Orlando) 2020;34:100513. [Crossref] [PubMed]
- Gomez-de-Antonio D, Campo-Cañaveral JL, Crowley S, et al. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J Heart Lung Transplant 2012;31:349-53. [Crossref] [PubMed]
- Suberviola B, Mons R, Ballesteros MA, et al. Excellent long-term outcome with lungs obtained from uncontrolled donation after circulatory death. Am J Transplant 2019;19:1195-201. [Crossref] [PubMed]
- Valdivia D, Gómez de Antonio D, Hoyos L, et al. Expanding the horizons: Uncontrolled donors after circulatory death for lung transplantation-First comparison with brain death donors. Clin Transplant 2019;33:e13561. [Crossref] [PubMed]
- Valenza F, Citerio G, Palleschi A, et al. Successful Transplantation of Lungs From an Uncontrolled Donor After Circulatory Death Preserved In Situ by Alveolar Recruitment Maneuvers and Assessed by Ex Vivo Lung Perfusion. Am J Transplant 2016;16:1312-8. [Crossref] [PubMed]
- Healey A, Watanabe Y, Mills C, et al. Initial lung transplantation experience with uncontrolled donation after cardiac death in North America. Am J Transplant 2020;20:1574-81. [Crossref] [PubMed]
- Levvey BJ, Harkess M, Hopkins P, et al. Excellent clinical outcomes from a national donation-after-determination-of-cardiac-death lung transplant collaborative. Am J Transplant 2012;12:2406-13. [Crossref] [PubMed]
- Cypel M, Yeung JC, Liu M, et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431-40. [Crossref] [PubMed]
- Valenza F, Rosso L, Coppola S, et al. Ex vivo lung perfusion to improve donor lung function and increase the number of organs available for transplantation. Transpl Int 2014;27:553-61. [Crossref] [PubMed]
- Machuca TN, Mercier O, Collaud S, et al. Lung transplantation with donation after circulatory determination of death donors and the impact of ex vivo lung perfusion. Am J Transplant 2015;15:993-1002. [Crossref] [PubMed]
- Fumagalli J, Rosso L, Gori F, et al. Early pulmonary function and mid-term outcome in lung transplantation after ex-vivo lung perfusion - a single-center, retrospective, observational, cohort study. Transpl Int 2020;33:773-85. [Crossref] [PubMed]
- Mohite PN, Sabashnikov A, García Sáez D, et al. Utilization of the Organ Care System Lung for the assessment of lungs from a donor after cardiac death (DCD) before bilateral transplantation. Perfusion 2015;30:427-30. [Crossref] [PubMed]
Cite this article as: Musso V, Righi I, Damarco F, Mazzucco A, Zanella A, Vivona L, Palleschi A. Lung donation after circulatory death. Curr Chall Thorac Surg 2023;5:9.