
Management of congenital isolated H-type tracheoesophageal fistula
Introduction
Congenital isolated (H-type) tracheoesophageal fistula (TEF) without esophageal atresia (EA) is a rare lesion. It occurs in about 4% of EA spectrum of anomalies and has an incidence of 1:50,000–80,000 births (1-3). Although it is widely known as H-type fistula, the actual course of the fistula is oblique. It extends from a cephalad opening on the posterior wall of the trachea to a more caudal position on the anterior esophageal wall (4). Hence, it resembles the letter “N” more than the letter “H”.
The first case to be reported in the English literature was described by Lamb in 1873 as cited by Andrassy et al. (5). The first successful surgical repair was reported in 1939 by Imperatori (6). A seven-year-old child after a long struggle with recurrent bouts of pulmonary infections and several irrelevant treatment attempts including tracheotomy was finally diagnosed as having H-type TEF only after a tracheoscopic evaluation. A successful TEF repair was performed thorough a tracheotomy.
Congenital H-type TEF may be found at any level from larynx to carina (7). In most reported series, the level of the fistula varied from C5 to T3 vertebra with at least 70% occurring at or above the level of the T2 vertebra (1,8,9). The level of the fistula is defined in relation to the carina in some reports and ranges from 1.5 to 4 cm above the carina (10). Most fistulas are single although there are reports of patients having more than one (11-13).
The fistula occurs in the posterior membranous portion of the trachea and because of the proximity of the adjacent esophagus, it is quite short. The lumen of the H-type TEF usually measures less than 1 cm. Larger fistulas can be seen and some of these communications extend throughout the length of the trachea. These latter defects are called laryngo-tracheo-esophageal clefts (1). Tracheoesophageal communications derive their epithelial lining from both tracheal and esophageal mucosa.
Signs and symptoms
The child suffering from H-type TEF elicits a characteristic clinical picture. The manifestations are apparent beginning in the first few days of life. Unexplained cyanotic spells with choking and coughing with feeding are always present. The coughing, choking and cyanosis during feeding is considered the classical “three Cs” of H-type fistulas by some authors (14,15). During feeding, esophageal content tends to move into the trachea under the pressure gradient generated by esophageal contractions (16). Liquids cause greater difficulty than solids. These symptoms are promptly relieved with nasogastric tube feeding.
Air escapes through the fistula into the esophagus and hence to the stomach with coughing or crying. This causes intermittent abdominal distension which is a readily recognizable clinical finding especially in neonates. Likewise, endotracheal intubation and intermittent positive pressure ventilation cause air leakage through the fistula into the stomach and abdominal distension can even be more prominent. In one report, presence of an H-type fistula was suspected because of bile observed in the endotracheal tube (17).
Recurrent bouts of pneumonia typically involving right upper lobe is observed in older infants and children. A high index of suspicion is necessary considering the diagnosis of H-type TEF in a newborn or an infant having typical symptoms and signs. Because the findings may be relatively subtle and are nonspecific, the diagnosis may be delayed into early childhood or even adulthood. It is apparent that the familiarity and the awareness of the neonatologists and pediatricians in a given center play a major role in the early recognition of the condition. In a report from a single center in Canada, the median age at diagnosis was 8 days and 13 (81%) of 16 patients were diagnosed in the neonatal period (14). However, in another report from Canada involving 14 tertiary children’s hospitals, the median age at surgery was 16.5 days and 32 (31%) out of 102 patients were diagnosed after 30 days of life (18). In a series involving 31 H-type fistula patients from China, only four (12.9%) were diagnosed before the age of 2 months and 12 (38.7%) were diagnosed after one year of age (19). The oldest patient reported to have congenital H-type TEF was 79 years old (20).
Although associated anomalies are generally regarded to be less common in H-type TEF cases than in other forms of EA anomalies, up to 69% of cases show evidence of VACTERL/CHARGE association (14).
Diagnostic tools
A proper diagnosis must firstly establish the presence of a TEF and secondly demonstrate its localization. Plain radiographs of the chest are not diagnostic but may be helpful. They may show evidence of aspiration pneumonitis and gastric distension that yield to a suspicion of H-type fistula especially in an infant with suggestive clinical findings.
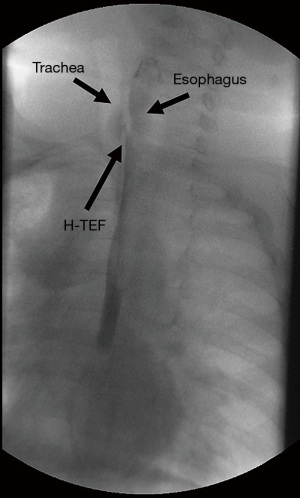
A video esophagography is commonly performed for diagnosing an H-type TEF (Figure 1). Although some authors claim a single contrast swallow esophagogram was diagnostic (14), more than 50% of the fistulas are missed on routine esophageal contrast studies in various studies (2,15). The clinical experience in many cases showed that repeat contrast studies might be needed to show the fistula (4,5,8,9,21). In one series, the initial contrast study enabled a positive diagnosis to be made in 20 (71%) of the 28 patients (9) and in 11 (47.8%) of the 23 patients in another study (15). An average of 1.4 esophagograms per patient were required for diagnosis in one series with eight patients (8). Coughing during the study may completely empty the contrast that has entered the trachea through the fistula. Moreover, normal active swallowing may not distend the esophagus sufficiently to allow the passage of the contrast into the TEF especially with the tendency of esophageal mucosa to occlude the fistula. It is of paramount importance that the study must be done by a radiologist familiar with the suspected H-type TEF diagnosis. For a proper study probably with a higher diagnostic yield, a “tube esophagogram” with “pullback technique” should be performed (4,8). For this purpose, first a nasogastric tube is passed into the distal end of the esophagus. Then, as the tube is withdrawn slowly the contrast medium is gradually injected under pressure with the infant in “prone Trendelenburg position” as suggested by Keats et al. in 1973 (22). Prone position is recommended because of the more caudal localization of the fistula on the esophageal side. The additive effect of gravity favors passage of the contrast material through the fistula in this position. Cineradiography must be obtained because spot films can miss the diagnosis. Because of higher localization of isolated H-type TEFs in comparison to those with associated EA, the contrast injection continues high up in the cervical esophagus increasing the risk of aspiration. Two cases out of five developed respiratory arrest at the time of tube esophagogram in one study (4). Therefore, contrast studies should be performed in a setting where resuscitation facilities are available.
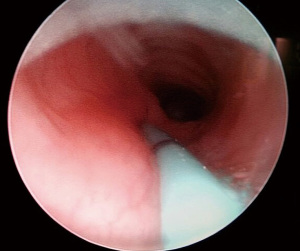
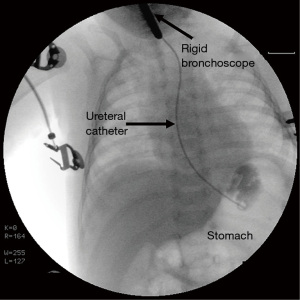
Endoscopic techniques can typically confirm the diagnosis (23). Bronchoscopy and esophagoscopy are complimentary techniques which can be used not only to identify but also to show the localization of the fistula which make them superior to radiological investigations. Esophagoscopy itself is not a priority diagnostic tool because the esophageal opening of the fistula is usually hidden in the folds of mucosa. Diagnostic bronchoscopy is definitely superior to esophagoscopy although in some rare instances the diagnosis was missed (4,16). Although rare, failure of bronchoscopic evaluation to show the fistula was thought to be related to causes like the close apposition of trachea and esophagus which may keep the fistula occluded most of the time and a spasm of the muscular layer with resultant occlusion of the lumen (16). The tracheal aspect of the fistula appears as a small, round, midline opening on the posterior membranous wall by bronchoscopy. Bronchoscopy enables passage of a catheter through the fistula as the fistula ascends cranially from the esophagus to the trachea (Figure 2). Direct radiography with contrast injection through the catheter may be obtained after cannulation (Figure 3). Esophagoscopy is used a s complementary measure like visualizing the catheter in the esophagus once bronchoscopic cannulation is done. It also enables assessing presence of an esophageal stenosis which is a rare but reported association (10,17,21,24). Bronchoscopic evaluation has the additional advantages of identifying associated tracheobronchial anomalies and also the rarely reported double fistulas. Among three reported cases with double congenital H-type fistulas, preoperative realization of a second fistula could be made in only one case although all three underwent preoperative diagnostic bronchoscopy (11-13). Therefore, all membranous portions of the trachea should be carefully inspected throughout its course during bronchoscopy.
There are some other diagnostic tools for H-type TEF. Myers suggested a “catheter test” if contrast studies fail to demonstrate the fistula in a patient with highly suggestive clinical signs (9). A catheter is passed into the esophagus and the end is placed under water. A vigorous bubbling occurring from the proximal end of the catheter suggest presence of fistula. As the authors implied, this test may yield to false positive results and is not widely used. A catheter with two balloons to occlude the proximal and distal esophagus was successfully used in a rabbit model (25). Until now, it has not been applied in humans. In another study, mediastinal ultrasonography was employed in 16 neonates with diseases in the EA spectrum none with H-type fistula (26). A small volume of saline solution was instilled into the blind upper esophageal pouch to document its extension. Ultrasonography revealed air bubbles moving across the mediastinum between the esophagus and trachea at two distinct levels in one infant with two proximal fistulas. However, ultrasonography has not gained wide acceptance in diagnosing these anomalies probably because it is an operator-dependent technique. Multidetector row computed tomography (MDCT) was diagnostic in three children aged 5, 9 and 15 years in whom both contrast studies and bronchoscopic evaluations had failed to show the H-type TEFs (16). The authors suggested MDCT with 3D imaging a non-invasive technique that renders fiberoptic bronchoscopy a confirmatory step to the diagnosis prior to surgery. However, this is a highly debatable argument considering the significant ionizing radiation related risks in children. Magnetic resonance imaging (MRI) was utilized to diagnose an H-type fistula in a baby weighing 880 grams with suggestive clinical signs despite a negative contrast study (27). The imaging was performed on a 1.5 T magnet using head and neck coil within 5 minutes and without anesthesia. T2-weighed images with a slice thickness of 2/0.5 mm were obtained and the TEF was demonstrated on axial images as a linear hypointense structure between trachea and esophagus at a distance 1 cm above the carina. In another study, high-resolution ultrashort echo time MRI was utilized to visualize tracheoesophageal anatomy in three newborns with distal TEFs associated with EA (28). Sedation or anesthesia was not needed in any. The technique was found to be safe and effective in visualization and evaluation of the anatomy in babies with EA and distal fistula. The potential use of MRI in H-type TEF remains to be elucidated in detail.
Treatment
Endoscopic treatment
The data about endoscopic treatment of congenital H-type TEF are limited. Bhatnagar et al. reported a case series for endoscopic treatment of TEF using electrocautery and the Nd:YAG laser in 1999 (29). Their series included two patients with congenital H-type TEF. The mucosal lining was fulgurated with electrocautery via an insulated wire with an unsuccessful outcome in one. It was vaporized with the Nd:YAG laser via a 600 micron bare quartz fiber with a successful outcome in the other. In a 2006 report, Tzifa et al. included two patients with H-type fistula in their series of TEF patients treated by diathermy followed by histoacryl tissue adhesive application (30). The patients with H-type fistulas were treated at the ages of 10 days and 4 weeks and they were closed in both with only one application. The authors concluded that the technique is safe and successful. On the other hand, in a later study from the same institution, treating primary TEF cases with endoscopy was reported to be an abandoned technique in their center (14). In 2016, Lelonge et al. reported 14 cases with TEF treated by rigid bronchoscopic chemo-cauterization with trichloroacetic acid (TCA) (31). Albeit none of the cases had H-type TEF, two had congenital proximal pouch TEF associated with EA; the remaining patients had recurrent fistulas. Cotton soaked with 50% TCA was applied on the TEF for 30 seconds and the procedure was repeated three times. Although the endoscopic treatment was performed monthly until TEF closure was achieved in the patients included in the series, one application successfully closed the fistulas in both congenital proximal pouch fistula cases.
Operative correction
Operative correction can be achieved by lateral cervical or thoracic approaches. The accurate estimation of the level of the fistula is essential to a satisfactory approach. Most congenital H-type TEF are characteristically rather high. It is near or above the thoracic outlet in many cases. Following the first successful repair of H-type TEF in 1939, a thoracic approach by thoracotomy was favored by many surgeons. Within a couple of decades, it was realized that transthoracic repair was “difficult” in most cases due to the high level of the fistula in the superior mediastinum or neck. In 1965, Killen reviewed the available literature and the results of repair according to operative approach showed that the thoracic approach carried higher rates of missing the fistula, recurrence and death (32). He emphasized the operative approach should be chosen in accordance with the level of the fistula and in most cases a cervical approach would be more appropriate.
The level of the fistula is usually defined relative to the vertebrae. The lesions located down to and including the T2 vertebra level are best approached by lateral cervical approach (7,19). The classical teaching dictates those below T2 vertebra level are most amenable to thoracic approach. However, some claim this as a dogma and even those fistulas as low as the carina can be successfully managed through a cervical incision (8,21,33). Anyway, most fistulas are located high up in the thorax regardless of the reference point about the level. In one study using sternal notch as a reference point, fistulas within 2 cm below the notch were deemed to be suitable for cervical repair (5). Only three (13%) of 23 H-type fistulas were more than 2 cm below the sternal notch. In another series which defined the level of the fistula in relation to the carina, the fistulas were located 1.5–2 cm above carina in four patients (50%), 3–3.5 cm above carina in three (37.5%) and 4 cm (12.5%) above carina in one out of a total of eight patients (10). They were all operated on by cervical approach.
In a 2014 review which included 17 studies with patients who underwent open surgery, cervical approach was utilized in 82 (90.1%) cases and thoracotomy in 9 (9.9%) (3). In a 2016 multicenter study from four centers, 22 (95.6%) patients were treated by cervical approach and the remaining patient by thoracoscopy (15). In a 2017 review which included 101 patients from 14 tertiary centers, 96% were repaired by cervical approach and the rest by thoracic approach (18). There is only one study with more patients having distally located fistulas than the proximally located ones (19). In that study, the fistulas were located between C7 and T2 in 13 (42%) patients and between T3 and T4 in 18 (58%) and therefore thoracic approach was utilized more commonly than the cervical one.
Bronchoscopic cannulation before the operation
The coupling of the surgery with endoscopic investigation which allows fistula catheterization is highly recommended if not considered “mandatory” (4,8). Guide wires, Fogarty catheters or ureteric catheters have been used for cannulation in different series (8,10,21,23,34). Ko et al. described a simplified approach for fistula division by inserting a flexible guide wire into the fistula into the esophagus during bronchoscopy in a patient with a fistula at the T1–2 level (35). The other end of the wire was retrieved through the mouth with an esophagoscope. A gentle traction applied to the now U-shaped wire eased the operation. Atzori et al. used a similar technique in a patient with “deep thoracic H-type fistula” (23). They inserted a guide wire into the fistula by bronchoscopy and then withdrew the other end through the mouth by fluoroscopy. Thus, the fistula could be lifted upwards during surgery and a cervical repair was possible.
Cervical approach
At the level of the thoracic inlet, the esophagus lies slightly to the left of the midline. However, the fistula angles to the right. Therefore, a low-lying right cervical incision was preferred in most series. A right-sided approach has also the advantage of minimizing the risk of injuring the thoracic duct (1,14). Also, recurrent laryngeal nerve lies more laterally on the right side making it less vulnerable to injury (8). Yet, some authors prefer a left-sided approach because esophagus is slightly to the left of the trachea thus accessibility is increased for the right-handed surgeon (5,19,34). Only very rarely, an anterior cervical approach was used (10). Among 82 patients who underwent isolated TEF repair by a cervical incision, a right cervical approach was utilized in 70 (85.4%) patients and a left cervical approach in 12 (14.6%) according to a recent review (3).
The infant is placed with the head extended and turned to the opposite side. A curving incision in the skin line is made low in the neck from the midline. It extends laterally beyond the sternocleidomastoid muscle. The muscle is either dissected free and retracted posteriorly or the sternal head is divided. With a medial dissection, the carotid sheath is reached and retracted posteriorly. The trachea and the esophagus are identified by palpating the endotracheal and nasogastric tubes. A plane is developed between the trachea and the esophagus and the inferior thyroid artery and the middle thyroid vain may be divided for this purpose. The recurrent laryngeal nerve must be identified and preserved. The esophagus can be encircled with a sling to ease the identification of the fistula. However, the contralateral recurrent laryngeal nerve can be damaged by this maneuver. Identification of the fistula is the key step during the operation. A preoperative cannulation of the fistula may aid in identification. The dissection continues until an adequate length of esophagus and trachea can be cleared below the fistula. Traction stiches are placed through the inferior and superior extent of the fistula to prevent rotation of the esophagus after fistula division. Traction sutures are placed on the trachea as well. The fistula is divided in close proximity with the esophagus leaving additional tissue on the tracheal side to enable a secure tracheal closure. First the tracheal then the esophageal sides of the fistula are closed with single layer 6-0 or 5-0 absorbable sutures. Although any consistent data is lacking, interposing of muscle tissue, fat or mediastinal pleura between the two opposing suture lines is advocated by some surgeons to reduce the chance of recurrence (1,2,14).
Thoracic approach
Thoracic approach for H-type fistula repair is used when the fistula is located within the thorax which is a rare occurrence. It can be done by way of standard thoracotomy or thoracoscopy.
A right sided thoracotomy is preferred for the open approach and the thorax is entered thorough the 4th or 5th intercostal space (19). The rest of the operation is similar to EA repair with a proximal esophageal dissection. The first report about thoracoscopic repair of an H-type fistula was published in 2004 (36). A 2.470-gram premature baby with a fistula between T2 and T3 level underwent a successful thoracoscopic repair with a 4-trocar technique. Over the ensuing years increasing numbers of patients were treated by thoracoscopy and some think thoracoscopy is superior to cervical approach. Proponents of thoracoscopic repair claim access to the superior mediastinum through the chest is excellent with improved visibility of tissues enabling identification and preservation of critical structures like the vagus nerve and also confinement of the field of dissection to the zone of the fistula only without circumferential esophageal or tracheal dissection (37). Rothenberg’s 2017 series included six patients with H-type fistulas who were treated thoracoscopically irrespective of the fistula level (38). Using a three-port technique, two fistulas were divided and sutured, two were clipped and divided, and two were divided with a 5 mm stapler. The postoperative period was uneventful in five patients. A partial disruption of the tracheal repair was detected on the night of the surgery in one patient. A thoracoscopic re-exploration with repair and placement of an intercostal muscle flap was done with a successful outcome.
Postoperative complications
Although mortality due to H-type TEF repair itself is extremely rare, complications were reported in up to two thirds of patients (8,14,18). They are thought to be more common with thoracic approach yet, one review found no statistically significant difference between cervical and thoracic approaches (3).
The most common complication is recurrent laryngeal nerve dysfunction. It occurs in 15% to 50% of patients and thought to originate from cervical dissection (8,14,15,39,40). The true incidence is probably unknown because in most series postoperative vocal cord evaluation is not done routinely. In children with postoperative respiratory symptoms such as stridor, increased respiratory effort with or without respiratory distress which may indicate vocal cord dysmotility, early vocal cord evaluation should be considered. In one series two (25%) patients had hoarseness and three (37.5%) had fiberoptic evidence of cord dysfunction out of a total of 8 patients (8). The symptoms resolved completely within a few months after surgery in all and the authors suggested there was temporary neuropraxic injury or glottic compensation. Zani reported 8 (50%) patients with postoperative vocal cord paresis, 6 right-sided and 2 bilateral (14). Although only three (38%) showed recovery of vocal cord movement, all eventually became asymptomatic. Postoperative laryngoscopy was performed in 33 (33%) of 102 patients in a multicenter review and 22 had vocal cord dysfunction (18). Among those with vocal cord dysfunction, eight (22%) had vocal cord paresis, ten (45%) unilateral paralysis and four (18%) bilateral paralysis. A tracheostomy was required in nine (9%). In another series with nine H-type fistula patients, a right-sided vocal cord paralysis was observed in two (18%) and a bilateral paralysis in one (9%) who eventually needed tracheostomy (39). Spontaneous regression of vocal cord dysmotility was observed in all with unilateral paralysis. Because spontaneous improvement can be expected in majority of the patients, a primary treatment approach of “wait and see” with orotracheal intubation and steroid use is usually advocated. For persistent vocal cord dysfunction, a posterior cordotomy with CO2 laser can be considered.
Although recurrences may occur, they are uncommon. The reported incidence ranges from 0% to 14% (8,14,18,39). The recurrence is usually in the early postoperative period and can be repaired by using the same initial surgical approach either cervical or thoracic (14,38).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editor (Tutku Soyer) for the series “Tracheoesophageal Fistula” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-150/coif). The series “Tracheoesophageal Fistula” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Randolph JG. Esophageal atresia and congenital stenosis. In: Welch KJ, Randolph JG, Ravitch MM, et al. editors. Pediatric Surgery. Mosby-Year Book. St. Louis, 1984:682-96.
- Harmon CM, Coran AG. Congenital anomalies of the esophagus. In: Coran AG, Adzick NS, Krummel TM, et al. editors. Pediatric Surgery. Elsevier Saunders, Philadelphia, 2012:893-918.
- Parolini F, Morandi A, Macchini F, et al. Cervical/thoracotomic/thoracoscopic approaches for H-type congenital tracheo-esophageal fistula: a systematic review. Int J Pediatr Otorhinolaryngol 2014;78:985-9. [Crossref] [PubMed]
- Ng J, Antao B, Bartram J, et al. Diagnostic difficulties in the management of H-type tracheoesophageal fistula. Acta Radiol 2006;47:801-5. [Crossref] [PubMed]
- Andrassy RJ, Ko P, Hanson BA, et al. Congenital tracheoesophageal fistula without esophageal atresia. Am J Surg 1980;140:731-3. [Crossref] [PubMed]
- Imperatori CJ. Congenital tracheoesophageal fistula without atresia of the esophagus. Report of a case with plastic closure and cure. Arch Otolaryngol 1939;30:352-9. [Crossref]
- Holder TM, Ashcraft KW. Esophageal atresia and tracheoesophageal fistula. Ann Thorac Surg 1970;9:445-67. [Crossref] [PubMed]
- Brookes JT, Smith MC, Smith RJH, et al. H-type congenital tracheoesophageal fistula:University of Iowa experience 1985-2005. Ann Otol Rhinol Laryngol 2007;116:363-8. [Crossref] [PubMed]
- Myers NA, Egami K. Congenital tracheo-oesophageal fistula. Pediatr Surg Int 1987;2:198-211. [Crossref]
- Genty E, Attal P, Nicollas R, et al. Congenital tracheoesophageal fistula without esophageal atresia. Int J Pediatr Otorhinolaryngol 1999;48:231-8. [Crossref] [PubMed]
- Babbitt DP. Double tracheoesophageal fistula without atresia. NEJM 1957;257:713-4. [Crossref] [PubMed]
- Schulte T, Ankermann T, Claas A, et al. An extremely rare abnormality of a double tracheoesophageal fistula without atresia of the esophagus; a case report and review of the literature. J Pediatr Surg 2009;44:e9-12. [Crossref] [PubMed]
- Mattei P. Double H-type tracheoesophageal fistulas identified and repaired in 1 operation. J Pediatr Surg 2012;47:e11-3. [Crossref] [PubMed]
- Zani A, Jamal L, Cobellis G, et al. Long-term outcomes following H-type tracheoesophageal fistula repair in infants. Pediatr Surg Int 2017;33:187-90. [Crossref] [PubMed]
- Al-Salem AH, Al Mohaidly M, Al-Buainain HMH, et al. Congenital H-type tracheoesophageal fistula: a national multicenter study. Pediatr Surg Int 2016;32:487-91. [Crossref] [PubMed]
- Gardella C, Toma P, Sacco O, et al. Intermittent gaseous bowel distension: atypical sign of congenital tracheaoesophageal fistula. Pediatr Pulmonol 2009;44:244-8. [Crossref] [PubMed]
- Durakbasa CU, Oskayli MC, Ozatman E, et al. A single institutional experience with congenital H-type tracheoesophageal fistula. Dis Esoph 2019;Suppl 1:doz047.52.
- Fallon SC, Langer JC, St Peter SD, et al. Congenital H-type tracheoesophageal fistula: A multicenter review of outcomes in a rare disease. J Pediatr Surg 2017;52:1711-4. [Crossref] [PubMed]
- Dai J, Pan Z, Wang Q, et al. Experience of diagnosis and treatment of 31 H-type tracheoesophageal fistula in a single clinical center. Pediatr Surg Int 2018;34:715-9. [Crossref] [PubMed]
- Garand SA, Kareti LR, Dumont TM, et al. Thoracoscopic repair of tracheoesophageal fistula in a septuagenarian. Ann Thorac Surg 2006;81:1899-901. [Crossref] [PubMed]
- Crabbe DCG, Kiely EM, Drake DP, et al. Management of the isolated congenital tracheo-oesophageal fistula. Eur J Pediatr Surg 1996;6:67-9. [Crossref] [PubMed]
- Keats TE, Smith TH. An improved positional technique for radiologic demonstration of infantile tracheoesophageal fistulae: a technical note. Radiology 1973;109:727. [Crossref] [PubMed]
- Atzori P, Iacobelli BD, Bottero S, et al. Preoperative tracheobronchoscopy in newborns with esophageal atresia: does it matter? J Pediatr Surg 2006;41:1054-7. [Crossref] [PubMed]
- Benjamin B, Pham T. Diagnosis of H-type tracheoesophageal fistula. J Pediatr Surg 1991;26:667-71. [Crossref] [PubMed]
- Kiyan G, Dagli TE, Tugtepe H, et al. Double balloon esophageal catheter for diagnosis of tracheo-esophageal fistula. Eur Radiol 2003;13:397-9. [Crossref] [PubMed]
- Gassner I, Geley TE. Sonographic evaluation of oesophageal atresia and tracheo-oesophageal fistula. Pediatr Radiol 2005;35:159-64. [Crossref] [PubMed]
- Gunlemez A, Anik Y, Elemen L, et al. H-type tracheoesophageal fistula in an extremely low birth weight premature neonate: appearance on magnetic resonance imaging. J Perinatol 2009;29:393-5. [Crossref] [PubMed]
- Higano NS, Bates AJ, Tkach JA, et al. Pre- and post-operative visualization of neonatal esophageal atresia/tracheoesophageal fistula via magnetic resonance imaging. J Pediatr Surg Case Rep 2018;29:5-8. [Crossref] [PubMed]
- Bhatnagar V, Lal R, Sriniwas M, et al. Endoscopic treatment of tracheoesophageal fistula using electrocautery and the Nd:YAG laser. J Pediatr Surg 1999;34:464-7. [Crossref] [PubMed]
- Tzifa KT, Maxwell EL. Endoscopic treatment of congenital H-Type and recurrent tracheoesophageal fistula with electrocautery and histoacryl glue. Int J Pediatr Otorhinolaryngol 2006;70:925-30. [Crossref] [PubMed]
- Lelonge Y, Varlet F, Varela P, et al. Chemocauterization with trichloroacetic acid in congenital and recurrent tracheoesophageal fistula: a minimally invasive treatment. Surg Endosc 2016;30:1662-6. [Crossref] [PubMed]
- Killen DA, Greenlee HB. Transcervical repair of H-type congenital tracheo-esophageal fistula. Ann Surg 1965;162:145-50. [Crossref] [PubMed]
- Hays DM, Wooley MM, Snyder WH. Esophageal atresia and tracheoesophageal fistula: management of the uncommon types. J Pediatr Surg 1966;1:240-52. [Crossref] [PubMed]
- Karnak İ, Şenocak ME, Hiçsönmez A, et al. The diagnosis and treatment of H-type tracheoesophageal fistula. J Pediatr Surg 1997;32:1670-4. [Crossref] [PubMed]
- Ko BA, Frederic R, Di Tirro PA, et al. Simplified access for division of the low cervical/high thoracic H-type tracheoesophageal fistula J Pediatr Surg 2000;35:1621-2. [Crossref] [PubMed]
- Allal H, Montes-Tapia F, Andina G, et al. Thoracoscopic repair of H-type tracheoesophageal fistula in the newborn: a technical case report. J Pediatr Surg 2004;39:1568-70. [Crossref] [PubMed]
- Lisle RM, Nataraja RM, Mahomed AA. Technical aspects of the thoracoscopic repair of a late presenting congenital H-type fistula. Pediatr Surg Int 2010;26:1233-6. [Crossref] [PubMed]
- Rothenberg SS. Thoracoscopic management of non-type C esophageal atresia and tracheoesophageal atresia. J Pediatr Surg 2017:S0022-3468(17)30647-4.
- Conforti A, Iacusso C, Valfrè L, et al. Cervical repair of congenital tracheoesophageal fistula: Complications lurking! J Pediatr Surg 2016;51:1623-6. [Crossref] [PubMed]
- Fung SW, Lapidus-Krol E, Chiang M, et al. Vocal cord dysfunction following esophageal atresia and tracheoesophageal fistula (EA/TEF) repair. J Pediatr Surg 2019;54:1551-6. [Crossref] [PubMed]
Cite this article as: Durakbasa CU. Management of congenital isolated H-type tracheoesophageal fistula. Curr Chall Thorac Surg 2022;4:23.


