
Review of the learning curve of video-assisted thoracic surgery & robotic-assisted thoracic surgery lobectomies—similarities and differences
Introduction
In the last two decades, thoracic surgery was at the center of a radical transformation: technological innovations in the surgical field gave numerous advantages in the treatment of lung cancer. Surgery of the lung is maybe the most representative example of this new scenario that permitted a radical change of our surgical approach which is now characterised by a minimally invasive treatment in most cases. Several recent international guidelines suggest a minimally invasive approach for lung cancer, especially in the early stages. Market introduction of thoracoscopes and cameras with higher image quality, an augmented reality perception through 3D vision and robotic assistance in surgery permitted the beginning of a new era in thoracic surgery.
Video-assisted thoracic surgery (VATS) and robotic-assisted thoracic surgery (RATS) are two different types of surgical approaches for lung cancer. Although VATS and RATS have been around for several years, they have undergone multiple improvements. VATS lobectomy began as a multiport approach with a service mini-thoracotomy with Roviaro’s pioneering experience in 1991 (1). Various approaches were evaluated through the years: varying the number of ports or shifting the approach from posterior to anterior, arriving at the uniportal approach as described by Gonzalez in 2010 (2).
Robotic surgery in pulmonary resections had a slower spread than mediastinal surgery (3). Improvement in imaging quality, freedom of movements and the reduction of physiological hand tremor represent the main points of the RATS evolution (4). This growth started with the first robotic pulmonary lobectomy performed by Melfi et al. in 2001 with two thoracoscopic accesses and a service mini-thoracotomy (5). Most recently, Cerfolio and Melfi again described a four-arm technique with an assistant port (6,7) and Turner a three-arm procedure with an assistant port (8).
Surgical evolution has therefore been accompanied by the need to bring surgeons closer to a change in their surgical technique in favour of the adoption of these minimally invasive techniques that were still held back by training limits in many centres. Only 6 years ago, Cao reported an interesting survey (Cross-sectional Survey on Lobectomy Approach; X-SOLA) studying a large cohort of lobectomy-performing thoracic surgeons to examine their adoption of VATS lobectomy: almost half of the sample perform lobectomy through a thoracotomy, but 92% of these surgeons responded that they were willing to learn videothoracoscopic technique, but were hindered by limited resources, exposure, and mentoring. They agreed there was a need for VATS lobectomy training in thoracic residency programs and in standardised workshops (9).
The need for training, reported by Cao, is what led many thoracic surgery centres to study how the learning of these new techniques took place among their surgeons with a statistical point of view. If we graphically represent the training of a surgeon to obtain efficiency with a new minimally invasive technique, we can see that an increase in his learning comes from a greater experience: the so-called learning curve (LC).
This review is aimed to evaluate not only the recent literature about the LC of VATS and RATS lobectomies but also what are, from our point of view, the similarities and differences between the two techniques.
Methods
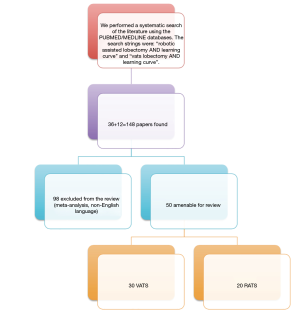
We performed a systematic search of the literature using the PUBMED/MEDLINE databases. The search strings were: “robotic assisted lobectomy AND learning curve” and “VATS lobectomy AND learning curve”. Additional studies were retrieved through a review of the references listed in the retrieved studies. We found a total of 148 papers (36 for RATS e 112 for VATS); we excluded 98 because metanalysis or non-English language; so we chose 50 reports published in the last 12 years, between 2008 and 2019 and are distributed as follows: 30 studies about the LC of VATS lobectomy (8 for uniportal technique and the remaining 22 for a general thoracoscopic approach with 2 or 3 ports) and 20 studies about the LC of RATS lobectomy (Figure 1).
The LC of VATS lobectomy
A consolidated experience of minimally invasive thoracic surgery for minor procedures such as pleural biopsy or pulmonary wedge resection allows the surgeon to develop confidence with correct port placement, use of video screen for guidance without looking through an access incision and use of an instrument designed for VATS (10).
In the revised papers we noticed that most of the authors studied their LC by dividing the patients who underwent a VATS lobectomy according to the chronological sequence of surgery. Vieira and Liu describe, for example, 3 phases: respectively these were the initial (first 60 lobectomies), the transitional [61-140] and the proficient phase [141-274] for the first author and the ascending (first 30 lobectomies), the plateau [31-60] and the descending phase [61-120] for the second one (11,12). With the same statistical division, Divisi and Zhao report their LC of VATS by dividing their lobectomies into 3 uniform groups based on their initial or late experience (13,14). On the other hand, many authors like Yao, Bedetti, Martin-Ucar, Gonfiotti and Cheng divide their cases into 2 groups: at the beginning and at the end of their period of study (15-19). Even if the distribution of the cases in the various phases reported in literature is quite uniform in several reports, we found some differences regarding the number of lobectomies necessary for good learning: Zhao reports that a surgeon becomes more proficient after 30-60 VATS lobectomies (14); Gonfiotti, Petersen, and Amore suggest a LC of 50 (18,20,21) while Yao, Cheng and Nachira refer 26, 28 and 25 lobectomies respectively as a LC for VATS (15,19,22). Instead, a greater number of cases are reported by Xiao Li who suggests that between 100 and 200 lobectomies are required to achieve efficiency while consistency requires even more cases (23). Mazzella also reports a bimodal distribution of the LC: oncological quality of the procedure improved and stabilised after 30 lobectomies but the surgeon obtained efficiency only after 90 cases (24).
In this review, the authors that we analysed often used the same parameters to evaluate their LC: operation time, blood loss, conversion rate to thoracotomy, number of dissected lymphadenopathy, postoperative complications rate, duration of chest drainage and subsequent length of stay, number of staplers attempted and need for an accessory thoracoscopic port in the LC of uniportal VATS. During the LC we found that it is common to show a reduction of the operative time, a reduction of blood loss and a less rate of conversion. Smith emphasised the impact of the LC and the preoperative factors which could determine an unplanned VATS conversion; he concludes that surgeons should be expected to perform a conversion to thoracotomy in patients who present tumours >3 cm (25). Unexpectedly, it was not uncommon to see an increase in the length of stay in some LC. This can be explained by the fact that the surgeon, past the initial learning phase, tends to become less selective with patients, performing more complex cases (adhesions, post-chemotherapy, incomplete fissure) that may favour a higher complication rate such as air leak that requires a major duration of chest drainage and a subsequent higher length of recovery in hospital.
Many authors evaluated if the surgeon’s previous experience has a positive influence on LC (17). Gezer investigated the case number required for gaining technical proficiency by applying cumulative sum analysis on initial VATS lobectomy operations of a single surgeon. He concludes that the length of LC depends on previous experience of the surgeon in open lobectomy and simpler VATS operations (26).
Contrarily, Okyere reports some papers that made direct comparisons between the outcomes achieved by trainees and fully qualified surgeons with prior experience in open lobectomy. Observing there was no increase in mortality in the trainee groups, he suggests that surgeons with limited experience in open lobectomy can achieve good outcomes in VATS lobectomy compared to their more experienced seniors (27).
In conclusion, we report what some authors suggest to hasten the LC. Jensen, for example, made a random selection of 28 surgical residents to either virtual-reality training or traditional black-box simulator training; they performed a VATS lobectomy on a porcine model after a retention period and their performance was scored: the performance of the black-box group was significantly faster than the virtual-reality group and no differences existed between the 2 groups when comparing bleeding and anatomical errors. The author confirms simulation-based training enabled the trainees to perform a simulated thoracoscopic lobectomy (28). Divisi suggests an interesting motto: “observing and doing for learning” is better than the old concept “observing and learning” advising the adoption of simulation programs (black-boxes, wet labs, cadaver or animal labs, 3D virtual reality simulators) (29). De La Torre and Hernandez-Arenas believe it is essential for the surgeon to implement his training in a high-volume training center because it allows surgeons to reach an expert level faster and to perform more complex resections with shorter training times (30,31). Another example is by Nachira, stressing the importance of attending dedicated courses (at least 2) for improving technique and shorten the LC, the importance to select the cases carefully, to standardise the technique and operating in two consultant surgeons (22). In opposition to the last Nachira’s suggestion, we found a very interesting teaching technique reported by Tcherveniakov: two registrars were scrubbed for each case (alternating as first surgeon and assistant) with the supervising consultant operating the camera. The author refers to the teaching process becomes more intuitive and is accelerated: this should reduce the LC considerably and improve safety during training (32). One last tip that we believe important to report is Vieira’s advice: perioperative and post-surgical outcomes should be recorded and audited regularly to allow adjustments during different learning phases. The author found that by watching systematically the video recordings of the surgeries one could understand which steps could be improved (11).
The LC of RATS lobectomy
In literature there are fewer reports about the LC of the RATS lobectomy if compared with VATS. This can be explained by two main reasons: a more recent introduction of robotic technique for lung lobectomy with Melfi in 2001 and fewer thoracic surgery centres performing robot-assisted operations. According to the basics of videothoracoscopy, special basic skills are also required to perform robotic lobectomy: instrument manipulation and clutching, 3D visualisation of the surgical field and camera control (33). Gharagozloo explains that four main factors can affect the LC of RATS lobectomy: a competent surgeon, a cumulative number of cases, a selection of ideal candidates for surgery (patients with minimal comorbidities, good pulmonary reserves and suitable disease characteristics) and the presence of a dedicated team (34). Besides, Veronesi and Seder add that other important positive features like no previous thoracotomy, no neoadjuvant chemotherapy, BMI < 40 Kg/m2, diameter maximum equal to 5 cm and no sleeve resections can be fundamental to become competent and obtain better results (35,36).
The Gharagozloo’s concept of a team dedicated to robotics plays a very important role. In robotic surgery, the first surgeon is not the only one to influence the trend of a LC: table-assistant must have manual dexterity and understand the steps of the operation because the operating surgeon should ideally remain at the console during the entire procedure (36). The anaesthesia team and nurses should be experienced in the management of double-lumen airway, but also competent in the robot setup, patient positioning and instrumentation (37). In a similar fashion to what we described for VATS lobectomies, we observed that most of the authors try to describe their LC in RATS lobectomies by dividing the patients of their studies into groups according to the time in which they were operated. Baldonado, for example, compared the outcomes of 2 patient groups who underwent robotic lobectomy divided by surgical date (38). Song and Arnold describe a LC evaluating the improvements in 3 groups of patients according to the chronological sequence of surgery (39,40). Current literature suggests that the LC needed to efficiently perform anatomic lung resections falls between 20 and 40 cases ranging from 14 cases as high as 60 (41). Meyer and Toker constructed a scatter plot to evaluate the relationship between the operative times and the extent of experience: the LC could be completed with 15 RATS lobectomies for the first author while 14 lobectomies are necessary for the second one in according with his study (42,43). Gharagozloo, Veronesi and Melfi proposed that the LC of RATS required 15 to 20 lobectomies (34,35,44).
The parameters taken into consideration during the LC by the authors analysed in this review, such as blood loss, rate of conversion, quality of lymphadenectomy or operative time, do not differ obviously from those assessed in the VATS. The docking time of the robot and body mass index (BMI) of the patients are the only different elements that some authors take into consideration in the analysis of their learning. Most of the authors reported a tendency to shorten operative times and to a decrease in conversion rate with greater experience. As seen for the LC of VATS, not many authors showed a decrease in terms of hospital stay while Veronesi reported there were no significant differences comparing early and experienced groups of patients in terms of lymph node dissection (35). Cao underlines that these eventualities mostly occurred in specialised centres having more than 30 cases (45).
Discussion
Reviewing these papers we can confirm that there are common cornerstones in the two LCs (Table 1). Reduction of operating times, reduction of blood loss, improvement of lymphadenectomy in terms of the number of dissected lymph nodes, reduction of the rate of conversion to thoracotomy and the reduction of the number of staplers attempted are all factors improved during a LC of VATS or RATS lobectomies. For VATS, a minimum number of at least 50 cases seems to be the most common among authors to overcome the first phase of the LC, ranging from 25 to 274 cases taking into account all the studies reviewed while in RATS numbers are smaller, ranging from 14 to 60 cases with an average of 20. For both surgical techniques, we frequently recorded the trend of an increasing length of stay justified by the fact that surgeons, after gaining competence, tend to operate more complex cases (adhesions, incomplete fissure, post-chemotherapy) and this facilitates a higher complication rate such as air leak which is what most influences the hospital stay. Regarding the differences between the two LCs, we noticed that some parameters have been taken into consideration in one of the minimally invasive techniques only. The need for accessory thoracoscopic access is particularly reduced with experience in LC of the single-port VATS. On the other hand, we found the evaluation of the docking time of the robot: the time needed to prepare the thoracoscope in VATS is not evaluated by any author, until now. We think that the robot itself represents a factor influencing the LC. In fact, many authors underline the importance to have basic skills first, for instrument manipulation and clutching, 3D visualisation of the surgical field and camera control.
Table 1
| Similarities | Differences |
|---|---|
| • Reduction of operating times | • The need for an accessory thoracoscopic access (only single-port VATS LC) |
| • Reduction of blood loss | • Docking time of the robot (RATS LC) |
| • Improvement of | • Instrument manipulation and clutching, 3D visualisation of the surgical field and camera control (RATS LC) |
| • Lymphadenectomy | • Body mass index (BMI) of the patients (RATS LC) |
| • Reduction of the rate of conversion to thoracotomy | |
| • Reduction of the number of staplers attemptes | |
| • Increasing length of stay |
LC, learning curve.
Another interesting point analysed in some reports about the LC of the RATS is BMI of the patients: some authors suggest operating patients with BMI <40 in the early stages of learning. We have not found this aspect in any of the reports analysed for the VATS.
Over the years, it seems that more and more authors decided to embrace the Divisi’s motto “observing and doing for learning”. Most of the papers suggest a good and an intense practice to learn better. Need to attend dedicated courses, adoption of simulation programs (black-boxes, wet labs, cadaver or animal labs, 3D virtual reality simulators) or training periods in centres with a high volume of cases are fundamental to improve and shorten the LC. We believe that it is more difficult to train a young surgeon in robotics than by videothoracoscopy: a limited number of hospitals can afford to have a double robotic console in the operating room. For this reason, learning is usually only for one surgeon at a time in this case. During a VATS lobectomy, more surgeons can be trained simultaneously. However, our observation seems to go against the trend of what is reported in the literature. Analysing the papers of this review, we found that the LC of the RATS was faster than VATS. Many authors report of 40 to 90 VATS lobectomies are necessary for correct learning. Comparing these numbers to the LC of 20–40 procedures found in the current review would suggest a more rapid LC for robotics. Considering that reports about RATS lobectomies studied the LC for surgeons with previous experience in VATS, it may be that the transition from performing open surgery to any minimally invasive approach requires more skill acquisition than transitioning from one minimally invasive approach (VATS) to a different minimally invasive approach (RATS).
Conclusions
VATS and RATS lobectomies are nowadays established as safe and effective procedures for lung cancer treatment as widely reported in the literature. We reviewed 50 papers about the LC of these two minimally invasive approaches published in the last ten years.
We observed a substantial overlap between the training for both techniques even though the LC of RATS seems to be shorter than VATS. This is probably explained by considering that RATS surgeons usually have a strong VATS background which implies easier learning of this approach. Independently, we believe that from the beginning to the end of a VATS or RATS lobectomy program two principles should be always followed: safe procedure for the patient and respect for the basic principles of oncological radicality. In conclusion, we can roughly identify three different periods of the minimally invasive thoracic surgery history. The first era, represented by the pioneering surgeons, who first conceived and demonstrated the validity of the 2 techniques. The second period, represented by a widespread diffusion of VATS and RATS which have been refined and improved by surgeons coming from the no minimally invasive surgery era. Therefore, we are looking towards the third period “to be”, in which young surgeons will grow up in a world of already established VATS and RATS approach to see how the LC will change.
Acknowledgments
Funding: None.
Footnote
Peer Review File: Available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-75/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form available at https://ccts.amegroups.com/article/view/10.21037/ccts-20-75/coif. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Roviaro G, Rebuffat C, Varoli F, et al. Videoendoscopic pulmonary lobectomy for cancer. Surg Laparosc Endosc. 1992;2:244-7. [PubMed]
- Gonzalez D, Paradela M, Garcia J, et al. Single-port video-assisted thoracoscopic lobectomy. Interact Cardiovasc Thorac Surg 2011;12:514-5. [Crossref] [PubMed]
- Zirafa CC, Romano G, Key TH, et al. The evolution of robotic thoracic surgery. Ann Cardiothorac Surg 2019;8:210-7. [Crossref] [PubMed]
- Ashrafian H, Clancy O, Grover V, et al. The evolution of robotic surgery: surgical and anaesthetic aspects. Br J Anaesth 2017;119:i72-84. [Crossref] [PubMed]
- Melfi FM, Menconi GF, Mariani AM, et al. Early experience with robotic technology for thoracoscopic surgery. Eur J Cardiothorac Surg 2002;21:864-8. [Crossref] [PubMed]
- Cerfolio RJ. Total port approach for robotic lobectomy. Thorac Surg Clin 2014;24:151-6. [Crossref] [PubMed]
- Melfi FM, Fanucchi O, Davini F, et al. VATS-based approach for robotic lobectomy. Thorac Surg Clin 2014;24:143-9. [Crossref] [PubMed]
- Turner SR, Latif MJ, Park BJ. Robotic assisted VATS lobectomy for loco-regionally advanced non-small cell lung cancer. Video Assist Thorac Surg. 2017;2:10. [Crossref] [PubMed]
- Cao C, Tian DH, Wolak K, et al. Cross-sectional survey on lobectomy approach (X-SOLA). Chest 2014;146:292-8. [Crossref] [PubMed]
- Hansen HJ, Petersen RH, et al. Video-assisted thoracoscopic lobectomy using a standardized three-port anterior approach - The Copenhagen experience. Ann Cardiothorac Surg 2012;1:70-6. [PubMed]
- Vieira A, Bourdages-Pageau E, Kennedy K, et al. The learning curve on uniportal video-assisted thoracic surgery: An analysis of proficiency. J Thorac Cardiovasc Surg 2020;159:2487-95.e2. [Crossref] [PubMed]
- Liu X, Chen X, Shen Y, et al. Learning curve for uniportal video-assisted thoracoscopic surgery lobectomy-results from 120 consecutive patients. J Thorac Dis 2018;10:5100-7. [Crossref] [PubMed]
- Divisi D, Bertolaccini L, Barone M, et al. National adoption of video-assisted thoracoscopic surgery (VATS) lobectomy: the Italian VATS register evaluation. J Thorac Dis 2018;10:330-8. [Crossref] [PubMed]
- Zhao H, Bu L, Yang F, et al. Video-assisted thoracoscopic surgery lobectomy for lung cancer: the learning curve. World J Surg 2010;34:2368-72. [Crossref] [PubMed]
- Yao F, Wang J, Yao J, et al. Video-Assisted Thoracic Surgical Lobectomy for Lung Cancer: Description of a Learning Curve. J Laparoendosc Adv Surg Tech A 2017;27:696-703. [Crossref] [PubMed]
- Bedetti B, Bertolaccini L, Solli P, et al. Learning curve and established phase for uniportal VATS lobectomies: the Papworth experience. J Thorac Dis 2017;9:138-42. [Crossref] [PubMed]
- Martin-Ucar AE, Aragon J, Bolufer Nadal S, et al. The influence of prior multiport experience on the learning curve for single-port thoracoscopic lobectomy: a multicentre comparative study. Eur J Cardiothorac Surg 2017;51:1183-7. [Crossref] [PubMed]
- Gonfiotti A, Bongiolatti S, Borgianni S, et al. Development of a video-assisted thoracoscopic lobectomy program in a single institution: results before and after completion of the learning curve. J Cardiothorac Surg 2016;11:130. [Crossref] [PubMed]
- Cheng YJ. The learning curve of the three-port two-instrument complete thoracoscopic lobectomy for lung cancer-A feasible technique worthy of popularization. Asian J Surg 2015;38:150-4. [Crossref] [PubMed]
- Petersen RH, Hansen HJ. Learning curve associated with VATS lobectomy. Ann Cardiothorac Surg 2012;1:47-50. [PubMed]
- Amore D, Curcio C. Steps in the development of a VATS lobectomy program. J Vis Surg 2017;3:104. [Crossref] [PubMed]
- Nachira D, Meacci E, Porziella V, et al. Learning curve of uniportal video-assisted lobectomy: analysis of 15-month experience in a single center. J Thorac Dis 2018;10:S3662-9. [Crossref] [PubMed]
- Li X, Wang J, Ferguson MK, et al. Competence versus mastery: the time course for developing proficiency in video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg 2014;147:1150-4. [Crossref] [PubMed]
- Mazzella A, Olland A, Falcoz PE, et al. Video-assisted thoracoscopic lobectomy: which is the learning curve of an experienced consultant? J Thorac Dis 2016;8:2444-53. [Crossref] [PubMed]
- Smith DE, Dietrich A, Nicolas M, et al. Conversion during thoracoscopic lobectomy: related factors and learning curve impact. Updates Surg 2015;67:427-32. [Crossref] [PubMed]
- Gezer S, Avcı A, Türktan M, et al. Cusum analysis for learning curve of videothoracoscopic lobectomy. Open Med (Wars) 2016;11:574-7. [Crossref] [PubMed]
- Okyere S, Attia R, Toufektzian L, et al. Is the learning curve for video-assisted thoracoscopic lobectomy affected by prior experience in open lobectomy? Interact Cardiovasc Thorac Surg 2015;21:108-12. [Crossref] [PubMed]
- Jensen K, Ringsted C, Hansen HJ, et al. Simulation-based training for thoracoscopic lobectomy: a randomized controlled trial: virtual-reality versus black-box simulation. Surg Endosc 2014;28:1821-9. [Crossref] [PubMed]
- Divisi D, Barone M, Zaccagna G, et al. Video-assisted thoracoscopic surgery lobectomy learning curve: what program should be offered in a residency course? J Vis Surg 2017;3:143. [Crossref] [PubMed]
- DE LA Torre M. Uniportal VATS lobectomy. Minerva Chir 2016;71:46-60. [PubMed]
- Hernandez-Arenas LA, Lin L, Purmessur RD, et al. Uniportal video-assisted thoracoscopic early learning curve for major lung resections in a high volume training center. J Thorac Dis 2018;10:S3670-7. [Crossref] [PubMed]
- Tcherveniakov P, Bogdan C, Chaudhuri N, et al. Crossing the bridge to VATS lobectomy. Ann R Coll Surg Engl 2017;99:650-2. [Crossref] [PubMed]
- Gomez PP, Willis RE, Van Sickle KR, et al. Development of a virtual reality robotic surgical curriculum using the da Vinci Si surgical system. Surg Endosc 2015;29:2171-9. [Crossref] [PubMed]
- Gharagozloo F, Margolis M, Tempesta B, et al. Robot-assisted lobectomy for early-stage lung cancer: report of 100 consecutive cases. Ann Thorac Surg 2009;88:380-4. [Crossref] [PubMed]
- Veronesi G, Galetta D, Maisonneuve P, et al. Four-arm robotic lobectomy for the treatment of early-stage lung cancer. J Thorac Cardiovasc Surg 2010;140:19-25. [Crossref] [PubMed]
- Seder CW, Cassivi SD, Wigle DA, et al. Navigating the pathway to robotic competency in general thoracic surgery. Innovations (Phila) 2013;8:184-9. [Crossref] [PubMed]
- Campos JH. An update on robotic thoracic surgery and anesthesia. Curr Opin Anaesthesiol 2010;23:1-6. [Crossref] [PubMed]
- Baldonado JJAR, Amaral M, Garrett J, et al. Credentialing for robotic lobectomy: what is the learning curve? A retrospective analysis of 272 consecutive cases by a single surgeon. J Robot Surg 2019;13:663-9. [Crossref] [PubMed]
- Song G, Sun X, Miao S, et al. Learning curve for robot-assisted lobectomy of lung cancer. J Thorac Dis 2019;11:2431-7. [Crossref] [PubMed]
- Arnold BN, Thomas DC, Bhatnagar V, et al. Defining the learning curve in robot-assisted thoracoscopic lobectomy. Surgery 2019;165:450-4. [Crossref] [PubMed]
- Power AD, D'Souza DM, Moffatt-Bruce SD, et al. Defining the learning curve of robotic thoracic surgery: what does it take? Surg Endosc 2019;33:3880-8. [Crossref] [PubMed]
- Meyer M, Gharagozloo F, Tempesta B, et al. The learning curve of robotic lobectomy. Int J Med Robot 2012;8:448-52. [Crossref] [PubMed]
- Toker A, Özyurtkan MO, Kaba E, et al. Robotic anatomic lung resections: the initial experience and description of learning in 102 cases. Surg Endosc 2016;30:676-83. [Crossref] [PubMed]
- Melfi FM, Mussi A. Robotically assisted lobectomy: learning curve and complications. Thorac Surg Clin 2008;18:289-95. [Crossref] [PubMed]
- Cao C, Manganas C, Ang SC, et al. A systematic review and meta-analysis on pulmonary resections by robotic video-assisted thoracic surgery. Ann Cardiothorac Surg 2012;1:3-10. [PubMed]
Cite this article as: Paladini P, Meniconi F, Ghisalberti M, Luzzi L, Ligabue T, Leonibus LD, Corzani R. Review of the learning curve of video-assisted thoracic surgery & robotic-assisted thoracic surgery lobectomies—similarities and differences. Curr Chall Thorac Surg 2021;3:16.


