Uniportal thoracoscopic surgery for pulmonary arteriovenous malformations—report of technique and case series
Introduction
Pulmonary arteriovenous malformations (PAVM) are vascular anomalies with a direct communication between pulmonary arteries and veins, bypassing the normal intervening capillary bed. By the majority, they are congenital, with dyspnea, epistaxis or hemoptysis as common clinical symptoms (1).
PAVM has firstly been described by Churton at autopsy in 1897 (2), and since then they have been given a great variety of names including pulmonary arteriovenous aneurysms or fistulas, hemangiomas or cavernous angiomas of the lung, pulmonary telangiectasias, or, as already mentioned, pulmonary arteriovenous malformations (1). The latter is the most commonly used term and will therefore be used throughout the following text.
The diagnosis of PAVM is made almost twice as frequently in women as compared to men. The estimated overall incidence is 38 per 100,000 persons (3). In up to 69%, a correlation is seen between PAVM and hereditary hemorrhagic telangiectasia (HHT), also known as Osler-Weber-Rendu syndrome (1). Conversely, PAVMs are present in up to 46% of patients with HHT (1,4,5). Although they can potentially affect any part of the lung, there is a predominance of the lower lobes (53–70%). In terms of localization, they can be found anywhere in the lung or spread over its surface (1,6,7).
PAVM can increase in number and size over time (7), therefore playing a role as differential diagnosis for evaluation of pulmonary nodules or hypoxemia and oxygen desaturation based on right-to-left shunting (8). About 70% of patients with PAVM are symptomatic (1,7). Bypassing the pulmonary capillary bed results in right-to-left shunt and potentially causes a variety of hypoxemic symptoms like exercise intolerance, dyspnea, cyanosis, digital clubbing, murmurs or bruits or even chest pain or cough (1,7).
The most frequent neurologic complications are transient ischemic attack, stroke or brain abscess and are most likely caused by paradoxical embolization based on the absence of a normal capillary bed, which normally acts as a filter for potential paradoxical embolisms (1,7,9). Rupture of PAVM can also lead to hemoptysis (endobronchial or intraparenchymal PAVM) or even hemothorax (e.g., superficial PAVM). Such bleedings can be life-threatening.
Diagnostic options are contrast enhanced CT scan of the chest or digital subtraction pulmonary angiography, measurement of shunt fraction (e.g., by 100% oxygen method) and contrast echocardiography (detection of right-to-left shunts) (1,10,11). Avoiding ionizing radiation, contrast-enhanced MR perfusion imaging and MR angiography are other alternative diagnostic modalities (12,13).
The main treatment goal of PAVM includes prevention of neurologic complications (due to paradoxical embolization), progressive hypoxemia with its resulting complications (high-output cardiac failure) and hemorrhage (hemoptysis and/or hemothorax). Current guidelines recommend treatment of PAVM with a feeding artery diameter of 2 mm or larger (6,7,14-19). For smaller feeding arteries, the decision should be made on an individual basis (6,14-18), because these lesions also have a growth potential and pose a risk of enlargement as well as embolic complications at a later time (6,15,17-19).
The first ‘successful’ treatment of a patient with a PAVM was a pneumonectomy, performed by Shenstone in 1940 in a case, which was diagnosed by Hepburn and Dauphinée and published by them two years later in 1942 (20,21). Before Taylor published the first interventional treatment by using a device for embolization of PAVM in 1978 (22), conventional open thoracic surgery was the only known treatment option.
Being less invasive than conventional open thoracic surgery and easy to repeat, thenceforward the interventional approach with percutaneous embolization using for example coils replaced surgery in treatment of patients suffering from PAVM, thereby eventually being the most performed and recommended treatment for PAVM at present (6). All patients with PAVM should be evaluated by an interventional radiologist or angiologist for embolotherapy before being considered for surgery. Surgical resection of PAVM is indicated in patients who failed embolotherapy, develop serious bleeding complication despite embolotherapy, have intrapleural rupture of the PAVM or have untreatable contrast allergy and lesions not amenable to embolotherapy. Despite the fact that surgery has been mostly replaced by interventional options for treatment of PAVM in recent years, novel minimally invasive operative techniques advanced and offer a safe therapeutic option with low morbidity and mortality when surgery is necessary. Ongoing improvements in technique and instruments for video-assisted thoracoscopic surgery (VATS) have allowed lung surgery to become more minimally invasive with faster postoperative rehabilitation. Conventional three-port VATS is already well established for different types of lung resections. In recent years, uniportal VATS anatomical lung resection has become a new area of exploration in minimally invasive thoracic surgery. Uniportal VATS is a less invasive approach that allows even major thoracic operations to be performed through a single small incision of some centimeters. Consistent reports confirmed different advantages of uniportal VATS, e.g., reduced surgical trauma, decreased postoperative pain, faster rehabilitation, and improved patient satisfaction with a less invasive approach than conventional VATS, but also with no significant differences in safety at the same time (23-25).
Methods
Our technique for uniportal VATS anatomical lung resection:
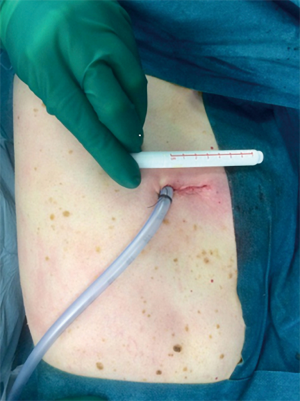
The procedure is performed under general anesthesia with a double-lumen endotracheal tube (DLT) or an endobronchial blocker. The patient is placed in a lateral decubitus position. The arms are flexed and positioned towards the head on two separate arm boards. The thoracic cavity is accessed by a 3–4 cm incision in the fourth or fifth intercostal space in the anterior to midaxillary line in a muscle-sparing technique (Figure 1). We routinely utilize a wound protector to maximize wound exposure, avoid soiling of the camera and protect the wound from contamination. No rib spreader is used. To explore the pleural cavity, a 5 mm 30° angled endoscope is used.
Vascular dissection can be performed analogous to any thoracoscopic anatomical resection. The targeted branches of the pulmonary artery are dissected and cut with endoscopic stapling devices prior to the vein, avoiding pressure dependent rupture of the pulmonary arteriovenous malformation. After dividing the artery, vein and bronchus, if deemed necessary, a systemic injection of indocyanine green (ICG) can be used in combination with near-infrared imaging to identify and mark the intersegmental planes.
Ethical approval was not required because of the descriptive manner of this study with limited participants (not more than 5). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from all patients for publication of this manuscript including accompanying images.
Patients
From October 2015 to August 2019, we performed uniportal VATS lung resections for PAVM in five patients. Four of these five patients underwent a lung-sparing uniportal VATS segmentectomy (3 single segmentectomies and one lingula resection) and in one patient, a uniportal wedge resection combined with embolotherapy was scheduled and performed for a complicated and extensive arteriovenous malformation between the superior phrenic artery and the right inferior pulmonary vein. Patient characteristics are summarized in Table 1.
Table 1
| Patient | Age (years) | Sex | Number of PAVM | Localization | HHT | Symptoms | Embolotherapy | Indication for surgery |
|---|---|---|---|---|---|---|---|---|
| A | 20 | F | 1 | RS8 | No | Dyspnea on exertion | Declined | Interdisciplinary discussion: patient prefers surgery |
| B | 57 | F | 1 | LS6 | No | Desaturation on exertion, stroke | – | Referral from cardiologist |
| C | 15 | M | 1 | RS6 | Yes | Epistaxis | – | Referral from otolaryngologist |
| D | 29 | F | 1 | Superior phrenic artery and right inferior pulmonary vein | No | Dyspnea on exertion | Unsuccessful, embolotherapy, decision for surgery combined with ethanol embolization afterwards | Interdisciplinary decision for combination after unsuccessful embolization |
| E | 37 | M | Multiple AVM | Lingula, RUL, both LL, brain | Yes | Epistaxis, chest pain, dyspnea, repetitive hemothorax | Coiling of PAVM in lingula plus preventive coiling of left bronchial artery during first hemothorax | Massive hemothorax |
PAVM, pulmonary arteriovenous malformations; AVM, arteriovenous malformation; RS8, right segment 8; LS6, left segment 6; RS6, right segment 6; RUL, right upper lobe; LL, lower lobe.
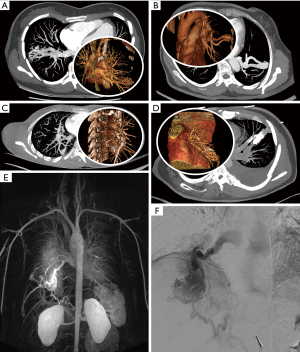
Three of five patients were female and in four patients PAVM was located in the lower lobe. One had recurrent disease with two PAVM in both lower lobes treated already in the past (Patient E). Two patients were diagnosed with hereditary hemorrhagic telangiectasia (C, E). Multiple lesions were only found in patient E, which have been previously treated by embolization in multiple sessions years before actual surgical treatment. The malformations of patients A, B, C and E could be accurately localized in one single lung segment or between two adjacent segments (lingula), well accessible by the uniportal minimal-invasive approach. Diagnostics included pulmonary function tests with respiratory measurement with exercise electrocardiogram, chest X-ray, computed tomography angiography (CTA) or magnetic resonance angiography (MRA) and evaluation for hereditary hemorrhagic telangiectasia (selected radiographic or angiographic findings are illustrated in Figure 2).
Clinical manifestations ranged from epistaxis without any respiratory symptoms (C) to hypoxemic symptoms and one stroke as the only neurologic complication in patient B. Patients A, B, D and E presented with dyspnea or oxygen desaturation on exertion. Additionally, patient E previously showed multiple clinical manifestations including epistaxis and chest pain due to hemothorax, following coiling of one bronchial artery and embolotherapy of the lingular PAVM with vascular plugs. Eight months later repetitive hemothorax occurred, presenting with ipsilateral chest pain, dyspnea and oxygen desaturation.
Indication for surgery was made based on number, size, distribution and localization of the malformations in an interdisciplinary setting.
Results
Five patients with pulmonary arteriovenous malformations were treated by uniportal minimally invasive thoracic surgery in the last four years. Performed resections, postoperative parameters and outcome including length of hospital stay, chest tube duration and follow-up time as well as complications and their management are listed in Table 2. Regarding the four solitary lesions which were completely located in the lung parenchyma, (A, B, C, E) anatomical resection of the involved single segment or both lingular segments in E was performed using the uniportal VATS approach reported before. In patient E, one additional and smaller ipsilateral PAVM, which has been coiled previously was left untreated because of stable size and missing signs of revascularization. The vascular confluence between the superior phrenic artery and the inferior pulmonary vein in patient D was locally excised right above the diaphragm and treated by embolization afterwards in a second step as planned preoperatively based on multidisciplinary clinical case discussion after unsuccessful embolization in a first step.
Table 2
| Patient | Operation | Drain removal (POD) | Discharged (POD) | Postoperative complications | Complication management | Follow-up |
|---|---|---|---|---|---|---|
| A | uVATS anatomical RS8 resection | 1 | 2 | None | – | Free of complaints at 52 months |
| B | uVATS anatomical LS6 resection | 1 | 5 | None | – | Free of respiratory complaints at 31 months, persistent amnesic aphasia since preoperative stroke |
| C | uVATS anatomical RS6 resection | 2 | 4 | Pneumothorax, subcutaneous emphysema | Conservative | Regressive airspace and emphysema, free of complaints at 5 months |
| D | uVATS wedge resection of vascular confluence in RLL & supradiaphragmatic dissection | 2 | 2 | None | – | Free of complaints at 8 months |
| E | uVATS anatomical lingula resection, evacuation of hematoma on the left | 3 | 5 | None | – | Increased numbers of AVM, no chest pain or dyspnea at 17 months |
PAVM, pulmonary arteriovenous malformations; AVM, arteriovenous malformation; POD, postoperative day; uVATS, uniportal video-assisted thoracic surgery; RS8, right segment 8; LS6, left segment 6; RS6, right segment 6; RLL, right lower lobe.
The median postoperative day (POD) for chest tube removal was two (range: 1 to 3) and patients were discharged between POD 2 and POD 5 (median: 4). In patient E with hemothorax as complication of PAVM, the chest tube was removed on POD 3 with discharge on POD 5.
There were no complications regarding PAVM during follow-up in all patients except in Patient E. In this patient with hereditary hemorrhagic telangiectasia, there were some new malformations found in other lobes than the operated one in CT scan follow-up 9 months after operation, without any complications or symptoms so far. Patient C showed a postoperative airspace slightly bigger than expected after segmentectomy with some subcutaneous emphysema after chest tube removal, which was successfully treated conservatively in an otherwise asymptomatic patient. Furthermore, the patient (D) who has undergone local excision of a malformation between the superior phrenic artery and the inferior pulmonary vein showed an elevated ipsilateral hemidiaphragm in the chest X-ray after tube removal as an incidental finding without any clinical complaints. No further diagnostics were made, since the patient was free of symptoms. There was a complete recovery in follow-up check-up. Overall follow-up period was up to 52 months postoperatively.
Discussion
Conventional open thoracic surgery has been replaced as preferred therapeutic option for PAVM by effective and less invasive interventional embolization techniques in the past, but in recent years thoracoscopic pulmonary surgery has also very much advanced (26). Technical feasibility, safety and advantages of VATS (27), especially for major anatomical lung resections, has been demonstrated in different meta-analyses (28,29), systematic reviews (30,31) and in prospective multi-institutional studies (32). Furthermore, even less invasive techniques have been developed in the last three decades. The technique of using a single small incision for VATS has revolutionized the way thoracic surgeons treat pulmonary diseases today (23,26). With the involvement of only one intercostal space and without using any rib spreading, single-incision video-assisted thoracoscopic anatomic resections of almost every extent can be performed without compromising safety compared to the traditional multi-portal approach. Furthermore, the uniportal VATS approach can potentially improve perioperative outcomes such as reduction of overall rate of complications, faster recovery with shorter length of hospital stay and shorter chest tube duration (24,25).
Although current guidelines (6,14-16,19) are still prioritizing embolotherapy, these recommendations and results originate from retrospective observational non-controlled case series and prospective cohort studies (6). At present, no direct comparison has been made between embolotherapy and minimal invasive surgical techniques such as any type of VATS in treatment of patients with PAVM.
Although there is no high level of evidence for surgery in treatment of PAVM, retrospective case reports and case series have shown good results (8,33-37).
In the literature mentioned above, indications for resection of the PAVM using a minimal invasive surgical approach are rather vague and no clear selection criteria have been formed so far. Indication for pulmonary resection as treatment of PAVM should be based on number, size, distribution and localization of the malformation and furthermore, should be an individual decision made by a multidisciplinary board. Moreover, patients’ preference and general health conditions may be respected as well.
The extent of surgical resection is determined by location and size of the lesion. Extra-anatomical wedge resection or anatomical resections such as segmentectomy, lobectomy or even pneumonectomy are possible types of resection (6). Lung transplantation is reported but not recommended (38,39). Multiple small PAVM can occur, persist or even new ones may be formed over time, especially in individuals with HHT, hence, neither embolization of detectable lesions nor surgery will be able to cure these patients and prevent them from developing new malformations (6). In these patients embolization might be considered over surgery due to avoidance of tissue loss after potential repetitive excision or resection of all detected lesions (6) (Appendix 1).
It is important to emphasize that the indication for treatment of individuals with PAVM should be based on multidisciplinary board recommendations and each case should be discussed separately to provide the most beneficial treatment option for every patient.
If no contraindications are present, interventional embolization approaches or combinations with embolotherapy and surgery as shown in our patient D can be considered. In case of unsuccessful embolotherapy or if complications occurred, surgery offers a safe therapeutic alternative.
Thanks to huge changes in thoracic surgery and development of minimal invasive approaches, patients can benefit from these new surgical techniques and VATS might be even considered as equal therapy modality in selected cases. However, considering the limited evidence in the form of only retrospective studies and small case series, further studies are needed to investigate this issue.
Conclusions
In all of the presented cases, uniportal VATS was performed effectively. In selected cases of patients with PAVM with easily accessible lesions, in cases of unsuccessful embolization, or in complications, minimal invasive thoracic surgery approaches such as the uniportal VATS technique are safe and feasible therapeutic options, which are well tolerated especially in combination with lung sparing resections.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts.2020.04.01/coif). GJK serves as an unpaid editorial board member of Current Challenges in Thoracic Surgery from September 2019 to August 2021; PD serves as an unpaid editorial board member of Current Challenges in Thoracic Surgery from December 2019 to November 2021. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Ethical approval was not required because of the descriptive manner of this study with limited participants (not more than 5). Written informed consent was obtained from all patients for publication of this manuscript and any accompanying images. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gossage JR, Kanj G. Pulmonary arteriovenous malformations. A state of the art review. Am J Respir Crit Care Med 1998;158:643-61. [Crossref] [PubMed]
- Churton T. Multiple aneurysm of pulmonary artery. Br Med J 1897;1:1223.
- Nakayama M, Nawa T, Chonan T, et al. Prevalence of pulmonary arteriovenous malformations as estimated by low-dose thoracic CT screening. Intern Med 2012;51:1677-81. [Crossref] [PubMed]
- Fuchizaki U, Miyamori H, Kitagawa S, et al. Hereditary haemorrhagic telangiectasia (Rendu-Osler-Weber disease). Lancet 2003;362:1490-4. [Crossref] [PubMed]
- Kjeldsen AD, Oxhoj H, Andersen PE, et al. Prevalence of pulmonary arteriovenous malformations (PAVMs) and occurrence of neurological symptoms in patients with hereditary haemorrhagic telangiectasia (HHT). J Intern Med 2000;248:255-62. [Crossref] [PubMed]
- Hsu CC, Kwan GN, Evans-Barns H, et al. Embolisation for pulmonary arteriovenous malformation. Cochrane Database Syst Rev 2018;1:CD008017. [PubMed]
- Swanson KL, Prakash UB, Stanson AW. Pulmonary arteriovenous fistulas: Mayo Clinic experience, 1982-1997. Mayo Clin Proc 1999;74:671-80. [Crossref] [PubMed]
- Georghiou G. Pulmonary arteriovenous malformation treated by lobectomy. Eur J Cardiothorac Surg 2003;24:328-30. [Crossref] [PubMed]
- Akiyama S, Hanada S, Uruga H, et al. Hereditary hemorrhagic telangiectasia with pulmonary arteriovenous malformations and embolic strokes treated successfully with video-assisted thoracoscopic resection. Intern Med 2013;52:1091-4. [Crossref] [PubMed]
- Remy J, Remy-Jardin M, Giraud F, et al. Angioarchitecture of pulmonary arteriovenous malformations: clinical utility of three-dimensional helical CT. Radiology 1994;191:657-64. [Crossref] [PubMed]
- White RI, Pollak JS, Wirth JA. Pulmonary Arteriovenous Malformations: Diagnosis and Transcatheter Embolotherapy. J Vasc Interv Radiol 1996;7:787-804. [Crossref] [PubMed]
- Tellapuri S, Park HS, Kalva SP. Pulmonary arteriovenous malformations. Int J Cardiovasc Imaging 2019;35:1421-8. [Crossref] [PubMed]
- Ohno Y, Hatabu H, Takenaka D, et al. Contrast-enhanced MR perfusion imaging and MR angiography: utility for management of pulmonary arteriovenous malformations for embolotherapy. Eur J Radiol 2002;41:136-46. [Crossref] [PubMed]
- Faughnan ME, Palda VA, Garcia-Tsao G, et al. International guidelines for the diagnosis and management of hereditary haemorrhagic telangiectasia. J Med Genet 2011;48:73-87. [Crossref] [PubMed]
- Shovlin CL, Condliffe R, Donaldson JW, et al. British Thoracic Society Clinical Statement on Pulmonary Arteriovenous Malformations. Thorax 2017;72:1154-63. [Crossref] [PubMed]
- Chick JFB, Reddy SN, Pyeritz RE, et al. A Survey of Pulmonary Arteriovenous Malformation Screening, Management, and Follow-Up in Hereditary Hemorrhagic Telangiectasia Centers of Excellence. Cardiovasc Intervent Radiol 2017;40:1003-9. [Crossref] [PubMed]
- Shovlin CL, Jackson JE, Bamford KB, et al. Primary determinants of ischaemic stroke/brain abscess risks are independent of severity of pulmonary arteriovenous malformations in hereditary haemorrhagic telangiectasia. Thorax 2008;63:259-66. [Crossref] [PubMed]
- Pollak JS, Saluja S, Thabet A, et al. Clinical and anatomic outcomes after embolotherapy of pulmonary arteriovenous malformations. J Vasc Interv Radiol 2006;17:35-44; quiz 5. [Crossref] [PubMed]
- Trerotola SO, Pyeritz RE. PAVM embolization: an update. AJR Am J Roentgenol 2010;195:837-45. [Crossref] [PubMed]
- Adams WE, Thornton TF Jr, Eichelberger L. Cavernous Hemangioma of the Lung (Arteriovenous Fistula): Report of a Case with successful Treatment by Pneumonectomy. Arch Surg 1944;49:51-8. [Crossref]
- Hayward J, Reid L. Cavernous pulmonary telangiectasis. Thorax 1949;4:137-46. [Crossref] [PubMed]
- Taylor BG, Cockerill EM, Manfredi F, et al. Therapeutic embolization of the pulmonary artery in pulmonary arteriovenous fistula. Am J Med 1978;64:360-5. [Crossref] [PubMed]
- Gonzalez-Rivas D, Paradela M, Fernandez R, et al. Uniportal video-assisted thoracoscopic lobectomy: two years of experience. Ann Thorac Surg 2013;95:426-32. [Crossref] [PubMed]
- Harris CG, James RS, Tian DH, et al. Systematic review and meta-analysis of uniportal versus multiportal video-assisted thoracoscopic lobectomy for lung cancer. Ann Cardiothorac Surg 2016;5:76-84. [Crossref] [PubMed]
- Sihoe AD. Reasons not to perform uniportal VATS lobectomy. J Thorac Dis 2016;8:S333-43. [PubMed]
- Sihoe ADL. The Evolution of VATS Lobectomy. In: Cardoso P. editor. Topics in Thoracic Surgery. Croatia (Rijeka): Intech; 2011.
- Howington JA, Blum MG, Chang AC, et al. Treatment of stage I and II non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e278S-e313S.
- Yan TD, Black D, Bannon PG, et al. Systematic review and meta-analysis of randomized and nonrandomized trials on safety and efficacy of video-assisted thoracic surgery lobectomy for early-stage non-small-cell lung cancer. J Clin Oncol 2009;27:2553-62. [Crossref] [PubMed]
- Cheng D, Downey RJ, Kernstine K, et al. Video-assisted thoracic surgery in lung cancer resection: a meta-analysis and systematic review of controlled trials. Innovations (Phila) 2007;2:261-92. [Crossref] [PubMed]
- Whitson BA, Groth SS, Duval SJ, et al. Surgery for early-stage non-small cell lung cancer: a systematic review of the video-assisted thoracoscopic surgery versus thoracotomy approaches to lobectomy. Ann Thorac Surg 2008;86:2008-16; discussion 16-8.
- West D, Rashid S, Dunning J. Does video-assisted thoracoscopic lobectomy produce equal cancer clearance compared to open lobectomy for non-small cell carcinoma of the lung? Interact Cardiovasc Thorac Surg 2007;6:110-6. [Crossref] [PubMed]
- Swanson SJ, Herndon JE 2nd, D'Amico TA, et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol 2007;25:4993-7. [Crossref] [PubMed]
- Nagano M, Ichinose J, Sasabuchi Y, et al. Surgery versus percutaneous transcatheter embolization for pulmonary arteriovenous malformation: Analysis of a national inpatient database in Japan. J Thorac Cardiovasc Surg 2017;154:1137-43. [Crossref] [PubMed]
- Bakhos CT, Wang SC, Rosen JM. Contemporary role of minimally invasive thoracic surgery in the management of pulmonary arteriovenous malformations: report of two cases and review of the literature. J Thorac Dis 2016;8:195-7. [PubMed]
- Reichert M, Kerber S, Alkoudmani I, et al. Management of a solitary pulmonary arteriovenous malformation by video-assisted thoracoscopic surgery and anatomic lingula resection: video and review. Surg Endosc 2016;30:1667-9. [Crossref] [PubMed]
- Sano A, Tsuchiya T. Thoracoscopic surgery for multiple peripheral pulmonary arteriovenous fistulas. Ann Thorac Surg 2015;99:1808-9. [Crossref] [PubMed]
- Temes RT, Paramsothy P, Endara SA, et al. Resection of a solitary pulmonary arteriovenous malformation by video-assisted thoracic surgery. J Thorac Cardiovasc Surg 1998;116:878-9. [Crossref] [PubMed]
- Faughnan ME, Lui YW, Wirth JA, et al. Diffuse pulmonary arteriovenous malformations: characteristics and prognosis. Chest 2000;117:31-8. [Crossref] [PubMed]
- Shovlin CL. Pulmonary arteriovenous malformations. Am J Respir Crit Care Med 2014;190:1217-28. [Crossref] [PubMed]
Cite this article as: Flury DV, Kocher GJ, Lutz JA, Schmid RA, Dorn P. Uniportal thoracoscopic surgery for pulmonary arteriovenous malformations—report of technique and case series. Curr Chall Thorac Surg 2020;2:26.