
Exceptional third-time single-lung transplantation in a cystic fibrosis patient with previous pneumonectomy: a case report
Introduction
Lung retransplantation mostly indicated for chronic lung allograft dysfunction (CLAD) represents about 2.4% of all lung transplantations (LTx) performed annually throughout the world. (1) The survival of retransplanted patients is about 62% at 1-year, 49% at 3-year and 45% at 5-year meaning less than after primary LTx (2). The causes of these poorer outcomes are probably due to the more challenging technique and, usually, a more frequent hyperimmunisation of those patients. The literature data concerning third-time LTx are almost nonexistent with only 4 cases reported and no data available for long-term outcomes. However, in paediatric patients, the improved outcomes regarding life expectancy after LTx offer them the possibility to have further LTx during their life.
We report, in accordance with the CARE guidelines, an exceptional case of a third-time LTx done on a single lung because of previous pneumonectomy.
Case presentation
A cystic fibrosis 13-year-old female patient with DF508 homozygote mutation underwent a primary LTx because of respiratory failure requiring mechanical ventilation. Hyperimmunisation due to a massive transfusion for polytrauma during her childhood had been identified before that LTx justifying preoperative plasmapheresis. After an uneventful 6.5-year post-LTx period, a humoral rejection appeared that was treated by plasmapheresis with unfavourable evolution leading to lung retransplantation at 19.5 years old.
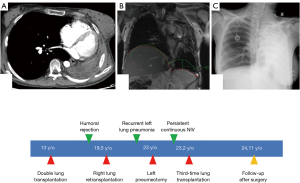
Unfortunately, only the right pulmonary graft was implanted because of an infracentimetric carcinoma discovered on the left graft after the right-side implantation. The early postoperative course was marked by temporary right hemidiaphragm paralysis associated with humoral and cellular rejection. Twenty-nine months after retransplantation, left graft pneumonectomy was performed to remove the destroyed left lung containing multiple infectious agents responsible for recurrent pneumonia. The postoperative course was marked by right-side pneumonia. Surprisingly, 2 months after surgery, the patient required continuous noninvasive ventilation. The aetiology of the respiratory disorder was not clear and 3 hypotheses were discussed. A post-pneumonectomy syndrome (PPS) with a reduction of the venous flow due to venous stretching (Figure 1A) following an extreme mediastinal shift was eliminated by echocardiogram. A diaphragm dysfunction was evoked because of previous temporary paralysis identified by Magnetic Resonance Imaging after retransplantation (Figure 1B). An electromyogram of the phrenic nerve showed good nerve conduction but no effective contraction of the hemidiaphragm who was flattened on chest X-ray but not really raised (Figure 1C), we supposed that the distended lung might impede its excursion. Finally, CLAD associated with near-diaphragmatic palsy secondary to lung distension was the retained diagnosis. Knowing that the 22-year-old patient could not be separated from invasive ventilation (she had a tracheotomy), the third-time LTx was proposed to the patient and her family to get out of that therapeutic impasse. No major hyperimmunisation was identified, which would have contraindicated LTx. Urgent priority LTx was accepted by experts because of persistent hypoxemia and hypercapnia under mechanical ventilation. Through median sternotomy, we performed an extended pneumolysis and positioned a mixed extracorporeal membrane oxygenation (ECMO)—peripheral arterial and central venous cannulations. The distended right lung compressing the diaphragm was removed and the new graft was implanted according to the usual surgical technique. ECMO weaning was possible at the end of the LTx and the postoperative course was uneventful. Diaphragm function rapidly recovered, the tracheostomy could be removed at day 30 and the patient was discharged from the hospital at day 40. Two months after the third-time LTx, the young woman did not need any more oxygen support. After twenty-three months she is still alive, in good condition, with a Forced Expiratory Volume in 1 second (FEV1) measured at 47% and she is back to normal life as an architecture student.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient and her legal guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Third-time LTx is a very rare procedure, even not considered under any circumstance in most countries, and we report a probably unique case performed after previous pneumonectomy in a Cystic Fibrosis patient. In 2007, Oto et al. (3) reported the case of a 46-year-old patient justifying a third-time LTx for CLAD 4.5 years after retransplantation. The authors emphasised the good clinical outcome by using extended criteria donor lungs from a Maastricht category IV donation but they did not give information about indication of primary LTx. Six months after surgery the patient was in good condition with FEV1 at 85% of predicted values. In 2008, Osaki et al. (4) reported 2 cases of third-time LTx for CLAD after primary LTx performed respectively for idiopathic pulmonary fibrosis and chronic obstructive pulmonary disease. The first patient died from multi-organ failure due to cecal rupture at postoperative day 86, and the other died at postoperative day 214 from pulmonary aspergillosis. The poor outcomes justified the authors’ conclusions for great caution and perhaps contraindication of such procedures. In 2011, Vakil et al. (5) reported the first case performed in a 25-year-old cystic fibrosis male patient. He underwent bilateral retransplantation 6 years after his primary LTx because of CLAD and, one year later, he had bilateral third-time LTx for the same indication. Twenty-four months after surgery, the patient was fine with FEV1 at 53%. In our case, before validating the surgical indication, we discussed several aspects: the potential severity of a third-time LTx in a young woman; the mechanisms involved to explain such major respiratory failure, and the particular case of LTx after previous contralateral pneumonectomy. Pneumonectomy and LTx are rarely associated and PPS occurs in 1% of cases after standard pneumonectomy but it was not implicated in our case. Diaphragm dysfunction after retransplantation was also evoked but ultrasound exam, routinely performed in intensive care unit, was delicate in our patient who permanently required noninvasive ventilation. So, the key exam was the diaphragm electromyogram identifying persistent conduction of the nerve, which did not result in effective muscle contractions. Finally, our multidisciplinary staff—including thoracic surgeons, pulmonologists, intensive care unit physicians, immunologists and anaesthesiologists—validated the diagnosis of severe CLAD associated with near-diaphragmatic palsy secondary to lung distension justifying a third-time LTx under urgent procedure. Twenty-three months later, the young woman went back to normal life without oxygen support comforting us in our decision.
In conclusion, we consider that the third-time LTx in that young woman was possible after rigorous brainstorming to understand the reasons for respiratory failure. We made the choice to accept the risks and the technical difficulties in order not to abandon this young patient who had a strong desire to live.
Acknowledgments
The authors are grateful to Valerie Mege Lin for editing English style and grammar.
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts.2019.12.11/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient and her legal guardian for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Yusen RD, Edwards LB, Kucheryavaya AY, et al. The registry of the International Society for Heart and Lung Transplantation: thirty-first adult lung and heart-lung transplant report--2014; focus theme: retransplantation. J Heart Lung Transplant 2014;33:1009-24. [Crossref] [PubMed]
- Kawut SM, Lederer DJ, Keshavjee S, et al. Outcomes after lung retransplantation in the modern era. Am J Respir Crit Care Med 2008;177:114-20. [Crossref] [PubMed]
- Oto T, Rowland M, Griffiths AP, et al. Third-time lung transplant using extended criteria lungs. Ann Thorac Surg 2007;84:642-4. [Crossref] [PubMed]
- Osaki S, Maloney JD, Meyer KC, et al. Redo lung transplantation for acute and chronic lung allograft failure: long-term follow-up in a single center. Eur J Cardiothorac Surg 2008;34:1191-7. [Crossref] [PubMed]
- Vakil N, Mason DP, Yun JJ, et al. Third-time lung transplantation in a patient with cystic fibrosis. J Thorac Cardiovasc Surg 2011;141:e3-5. [Crossref] [PubMed]
Cite this article as: Le Pimpec-Barthes F, Mangiameli G, Pricopi C, Arame A, Hernigou A, Guillemain R, Boussaud V. Exceptional third-time single-lung transplantation in a cystic fibrosis patient with previous pneumonectomy: a case report. Curr Chall Thorac Surg 2020;2:10.


