Giant posterior mediastinal schwannoma with ancient features: a case report
Introduction
The most common type of posterior mediastinal tumor is neurogenic and over 75% of these tumors are schwannomas (1). These masses arise from the sheaths of peripheral nerves, are completely encapsulated, and consist primarily of Schwann cells. In contrast, neurofibromas are tumors composed of Schwann cells, perineurial-like cells, fibroblasts, collagen and myxoid matrix. The intratumor nerve fibers present in neurofibromas help distinguish them from schwannomas, as the neoplastic cell of origin for both is the Schwann cell (2). Mediastinal schwannomas typically arise from the intercostal nerves, but may also arise from spinal, paravertebral sympathetic, vagus and phrenic nerves, as well as the thoracic nerve roots (3). These tumors are known to grow slowly and are usually an incidental finding in adults. Despite their insidious nature, schwannomas are primarily benign entities, and malignant transformation is exceptionally rare in adults (4). A variety of pathologies have been described for these benign neoplasms, including cellular, melanotic and psammomatous melanotic (which can become malignant), plexiform and ancient (2). Fine needle aspiration or biopsy often cannot provide a definitive histologic diagnosis to rule out malignancy (1). While biopsy may offer some reassurance of a benign neoplasm, surgical resection is indicated to confirm the diagnosis, obtain local control and reduce any present symptoms (3). Most reports in the literature describe schwannomas that are less than 10 cm. However, here we describe a 23-year-old female with a symptomatic 11×13 cm posterior mediastinal schwannoma with focal ancient features.
Case presentation
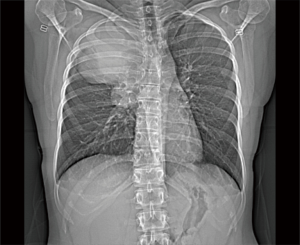
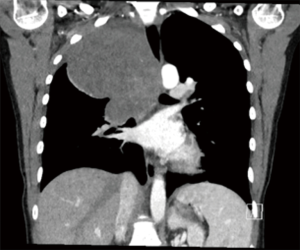
A 23-year-old non-smoking female with past medical history of scoliosis presented to her primary care provider with complaints of 2 years of intermittent chest heaviness and occasional tachycardia that was worse when lying on her right side. She denied any shortness of breath, difficulty breathing, or chest pain. She was able to lie flat on her back. She had no back pain or other neurologic symptoms. A chest X-ray showed a large apical mass in the right hemi-thorax (see Figure 1). Computed tomography (CT) of the chest with contrast demonstrated an 11×12×13 cm posterior apical mass which was possibly arising from the right upper lobe with mass effect on the mediastinal contents with resultant tracheal narrowing (see Figure 2). She was referred to thoracic surgery for further evaluation and treatment.
Physical exam revealed a healthy appearing female with normal vital signs, a patent airway without stridor, absence of tracheal deviation in the neck, and no evidence of supraclavicular or cervical adenopathy. The lung fields were clear to auscultation bilaterally, with decreased breath sounds at the right apex. Her heart rate and rhythm were normal. The remainder of her exam was unremarkable.
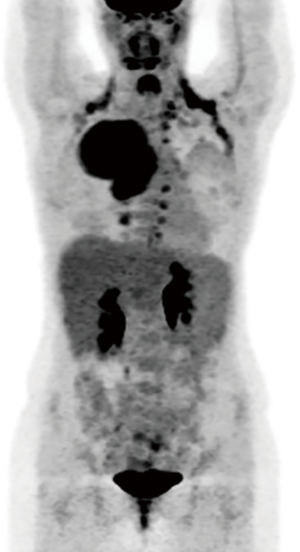
An electrocardiogram demonstrated normal sinus rhythm. Pulmonary function testing demonstrated a forced vital capacity of 4.12 L, 100% of predicted; a forced expiratory volume in 1 second of 2.35 L, 71% of predicted and a diffusing capacity of carbon monoxide of 97% of predicted. A brain magnetic resonance imaging scan was performed and showed no evidence of metastatic disease. A positron emission tomography (PET)/CT scan demonstrated findings consistent with a highly flourodeoxyglucose-avid right upper lobe mass extending to the pleural surface with deviation of the mediastinum to the left of midline (see Figure 3). There was a non-avid small associated pleural effusion. Additional avidity was felt to represent brown fat. There was no evidence of avid lymphadenopathy or aggressive periosteal changes along the corresponding posterior ribs or vertebral bodies to suggest invasion or unresectability.
At this point, the differential diagnosis included posterior mediastinal mass (likely neurogenic), primary lung cancer, metastatic cancer, or pleural based benign or malignant mass (e.g., benign fibrous tumor or malignant fibrous tumor of the pleura). The intense PET-avidity raised possible concern for underlying sarcomatous degeneration. Her case was reviewed at our multi-disciplinary comprehensive thoracic oncology program tumor board with the most likely diagnosis being a neurogenic tumor. The consensus recommendation was to rule out a metastatic or unresectable lung cancer via thoracoscopy and then proceed with resection for diagnosis and alleviation of symptoms via thoracotomy.
The patient underwent bronchoscopy, which revealed no endobronchial evidence of invasion into the trachea, but did show extrinsic compression on the right lateral aspect of the airway. A 5 mm thoracoscope was inserted into the right pleural space for direct visualization of the mass. This demonstrated the apical mass was separate from the lung and there was no evidence of intrapleural metastases. A right posterior-lateral serratus-sparing thoracotomy was performed. A 12-gauge core needle was used to biopsy several locations of the mass and sent for frozen sectioning. These demonstrated a bland spindle cell neoplasm with no obvious evidence of malignancy.
The mass was densely adherent to the anterior spinal ligament at the 4th and 5th vertebral bodies, and a portion of the ligament was resected en bloc with the mass. Circumferential dissection of the overlying pleura was performed. A single intercostal nerve coursed directly into the mass. This nerve was ligated with titanium clips and transected. There were some adhesions of the upper and lower lobes of the right lung to the mass. The lower lobe was able to be completely freed, but an area at the apex of the upper lobe had dense adhesions and a small apical wedge resection of the right upper lobe was required, utilizing an Endo-GIA stapler. There were significant adhesions to the mediastinal pleura as well. These were dissected free and showed no evidence of invasion into any of the mediastinal structures, including the phrenic nerve. The mass was completely mobilized and excised and a portion was sent for frozen sectioning. This was also negative for malignancy and similar to the core needle biopsies. However, given the large size of the mass, margins along the chest wall were taken as well. These were also negative for malignancy on intraoperative frozen section. She recovered well from surgery and was discharged home on post-operative day 3.
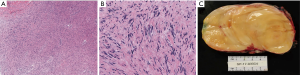
Final pathology revealed an encapsulated 12.3 cm schwannoma with focal ancient change with many Antoni B type areas and Verocay bodies (see Figure 4). Immunohistochemistry was positive for S100 and negative for epithelial membrane antigen (EMA) and neurofilament (NF) protein. Given the size of the mass, the multi-disciplinary consensus was to repeat a CT scan in 6 months, which showed no evidence of recurrence or new nodularity on the pleura or in the lung. At that time, she did note increased flushing and sweating of her left face and torso during exercise. She had no symptoms of Horner’s Syndrome. These symptoms are attributable to sympathectomy during the posterior dissection of the mass. At a follow-up visit 2 years post-resection, she continues to be free from recurrence but still notes reactive sympathetic stimulation of the left half of her face and torso, which minimally impacts her quality of life.
All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Although only 10% of Schwannomas occur in the mediastinum, they are the most common posterior mediastinal tumor (1). These encapsulated masses arise from peripheral nerve sheaths, and consist primarily of Schwann cells (4). Within the mediastinum, schwannomas typically arise from intercostal nerves, though there are a few reports of schwannomas originating from the vagus and phrenic nerves (1). Histologically, schwannomas have distinctive growth patterns consisting of hyper- and hypocellular areas of spindle cell proliferation, also known as Antoni A and Antoni B areas, respectively. The Antoni A type growth pattern is very hypercellular, consisting specifically of spindle cells organized in compact bundles, occasionally in the shape of Verocay bodies, which are areas of tightly organized nuclear palisades. In contrast, Antoni B areas are hypocellular, loosely arranged, consist of a copious myxoid matrix, and lack any specific histologic features (5,6). While proportions of Antoni A and B areas can vary greatly between tumors, this does not seem to carry prognostic significance. As a result of the spindle cell proliferation, these masses are profoundly positive for S100 on immunohistochemistry. Mitoses are rare in benign tumors and they are typically negative for pan-cytokeratin, cluster of differentiation (CD)-34, CD-117, calcitonin, smooth muscle actin, desmin, NF, and EMA (7). The fibrous capsule around the schwannoma usually contains the nerve from which the neoplastic process originated.
Multiple pathologic variants of schwannomas have been described (2). The cellular type demonstrates predominantly the Antoni A growth pattern and does not contain Verocay bodies. Melanotic schwannomas have a dense melanin pigmentation and, unlike the other types, have a predilection for malignant transformation. These melanotic variants can also contain psammoma bodies, which are areas of round, laminated calcium. Plexiform schwannomas tend to occur along plexuses of nerves and are aggregates of multiple tumors. These are rare and do not become malignant.
Schwannomas that are large and long standing may demonstrate areas of degenerative changes, including nuclear pleomorphism, hemorrhage, hyalinization, cyst formation and focal calcification, as well as a loss of Antoni A areas. These features are characteristic of “ancient schwannomas” (2,8). Consequently, these atypical features, which are thought to be attributed to the growth of the tumor, are often mistaken as areas of malignancy (6,9). Despite these changes, ancient schwannomas are not clinically distinct entities and some debate whether they should be considered a separate variant. As with other schwannomas, they are benign, slow-growing and typically asymptomatic until they become large enough to cause symptoms via compression on surrounding structures. They have an exceptionally low malignancy transformation and rarely recur following surgical excision (10,11).
While neurogenic tumors are more common in the pediatric population, schwannomas specifically are most common in adults between the ages of 20 and 70, with most occurring in adults 40 and over, and affect males and females equally (12). The malignancy rate of these tumors is higher within the pediatric population and the rate of malignant transformation decreases with age. Consequently, within adult populations these tumors are mostly benign (13). There is the potential for malignant transformation in adults, though this is rare (14). Typically, schwannomas are asymptomatic in adults and are consequently usually found incidentally on imaging tests (15). Despite their benign nature, they can lead to compressive pathology (16). This is typically most common with mediastinal schwannomas, with approximately 35% of patients symptomatic at the time of diagnosis (3,17,18). Previous descriptions note the average size of schwannomas to be approximately 3 cm with few reports >10 cm (19,20).
The only curative treatment for a schwannoma is complete surgical resection, which can be accomplished via video-assisted thoracoscopic surgery (VATS) or thoracotomy. The type of surgical approach is generally dependent on size and presence of intraspinal extension (21). However, violation of the capsule during resection should be avoided. It is estimated that only 10% of neurogenic mediastinal tumors extend in to the spinal canal. When extension is present, these are known as “dumbbell tumors” and despite intraspinal extension, 40% of these tumors will remain asymptomatic. When planning for resection of “dumbbell tumors”, a multi-disciplinary approach and consultation with a spine surgeon is often warranted. Adjuvant radiotherapy is typically only utilized for malignant schwannomas (22). Fortunately for all benign schwannomas, recurrence is extremely rare if complete surgical resection is achieved.
While prognosis after surgical resection remains positive, the challenge regarding schwannomas is the preservation of the nerve from which the mass originates. Typically, if the mass arises from an intercostal nerve, the nerve root may be sacrificed with few repercussions. However, if the mass originates from the vagus or phrenic nerves, care should be taken to limit any damage. The most common types of complications to occur as a result of nerve resection include Horner’s syndrome, partial sympathectomy, recurrent laryngeal nerve injury, and paraplegia (7). Horner’s syndrome is a constellation of symptoms, including miosis, ptosis and anhidrosis resulting from disruption to the sympathetic chain above the level of the stellate ganglion. For those that experience symptomatic schwannomas, Horner’s syndrome is often described as the presenting symptoms, usually as result of compression from the mass. However, Horner’s syndrome can also occur post-operatively due to damage incurred from resection (7,23). While symptoms of post-operative Horner’s syndrome were ruled out in the present case, she did present with symptoms of reactive sympathetic stimulation. Her symptoms included increased facial sweating and flushing on the contralateral side which occurred during exercise, and is a result of ipsilateral sympathectomy which occurred during resection of the mass. Compensatory sympathetic stimulation may present following division of the sympathetic chain, as seen in sympathectomy for hyperhidrosis.
Conclusions
Although most reports of mediastinal schwannomas are in older adults with an insidious course, this case describes a young adult with a symptomatic schwannoma greater than 10 cm in size over a 2-year period. While these tumors are typically benign, rare forms can have malignant degeneration. Complete resection for diagnosis, local control and reduction of symptoms is indicated.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Puma and Hon Chi Suen) for the series “Surgical Management of Chest Wall Tumors” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts.2019.12.13/coif). The series “Surgical Management of Chest Wall Tumors” was commissioned by the editorial office without any funding or sponsorship. JDP has received personal fees from Intuitive Surgical, Inc outside the published work and has no ongoing relationship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and national research committees and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Fierro N, D’ermo G, Di Cola G, et al. Posterior mediastinal schwannoma. Asian Cardiovasc Thorac Ann 2003;11:72-3. [Crossref] [PubMed]
- Skovronsky DM, Oberholtzer JC. Pathologic classification of peripheral nerve tumors. Neurosurg Clin N Am 2004;15:157-66. [Crossref] [PubMed]
- Ribet ME, Cardot GR. Neurogenic tumors of the thorax. Ann Thorac Surg 1994;58:1091-5. [Crossref] [PubMed]
- Tateishi U, Gladish GW, Kusumoto M, et al. Chest wall tumors: radiologic findings and pathologic correlation: part 1. Benign tumors. Radiographics 2003;23:1477-90. [Crossref] [PubMed]
- Kalhor N, Moran C. Neurogenic tumors. In: Kalhor N, Moran C. Mediastinal pathology. Berlin: Springer, 2019:399-454.
- Tahir MZ, Fatimi SH, Enam SA. Ancient schwannoma presenting as a thoracic mass. Surg Neurol 2007;68:534-6. [Crossref] [PubMed]
- Reeder LB. Neurogenic tumors of the mediastinum. Semin Thorac Cardiovasc Surg 2000;12:261-7. [Crossref] [PubMed]
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol 2004;52:229-39. [Crossref] [PubMed]
- Kara M, Ozkan M, Sak SD, et al. Giant ancient schwannoma of the posterior mediastinum cytologically misdiagnosed as a malignant tumour. A case report. Acta Chir Belg 2002;102:464-6. [Crossref] [PubMed]
- Kirschbaum A, Ritz R, Pehl A, et al. Giant intrathoracic left-sided vagal schwannoma. Thorac Cardiovasc Surg Rep 2013;2:19-22. [Crossref] [PubMed]
- Shanmugasundaram G, Thangavel P, Venkataraman B, et al. Incidental ancient schwannoma of the posterior mediastinum in a young male: a rare scenario. BMJ Case Rep 2019; [Crossref] [PubMed]
- Takeda S, Miyoshi S, Minami M, et al. Intrathoracic neurogenic tumors--50 years’ experience in a Japanese institution. Eur J Cardiothorac Surg 2004;26:807-12. [Crossref] [PubMed]
- Topçu S, Alper A, Gülhan E, et al. Neurogenic tumours of the mediastinum: a report of 60 cases. Can Respir J 2000;7:261-5. [Crossref] [PubMed]
- Woodruff JM, Selig AM, Crowley K, et al. Schwannoma (neurilemoma) with malignant transformation. A rare, distinctive peripheral nerve tumor. Am J Surg Pathol 1994;18:882-95. [Crossref] [PubMed]
- Duwe BV, Sterman DH, Musani AI. Tumors of the mediastinum. Chest 2005;128:2893-909. [Crossref] [PubMed]
- Strollo DC, Rosado-de-Christenson ML, Jett JR. Primary mediastinal tumors: part II. Tumors of the middle and posterior mediastinum. Chest 1997;112:1344-57. [Crossref] [PubMed]
- Bicakcioglu P, Demirag F, Yazicioglu A, et al. Intrathoracic neurogenic tumors. Thorac Cardiovasc Surg 2014;62:147-52. [PubMed]
- Loftus TJ, Pipkin M, Machuca T, et al. Angiographic embolization followed by piecemeal resection of giant posterior mediastinal schwannoma: case report and concise review. Int J Surg Case Rep 2018;53:250-3. [Crossref] [PubMed]
- Quartey B, Lenert J, Deb SJ, et al. Giant posterior mediastinal ancient schwannoma requiring thoracoabdominal resection: a case report and literature review. World J Oncol 2011;2:191-4. [PubMed]
- Knight DM, Birch R, Pringle J. Benign solitary schwannomas: a review of 234 cases. J Bone Joint Surg Br 2007;89:382-7. [Crossref] [PubMed]
- Cardillo G, Carleo F, Khalil MW, et al. Surgical treatment of benign neurogenic tumours of the mediastinum: a single institution report. Eur J Cardiothorac Surg 2008;34:1210-4. [Crossref] [PubMed]
- Kapoor A, Singhal MK, Narayan S, et al. Mediastinal schwannoma: a clinical, pathologic, and imaging review. South Asian J Cancer 2015;4:104-5. [Crossref] [PubMed]
- Kanagalingam S, Miller NR. Horner syndrome: clinical perspectives. Eye Brain 2015;7:35-46. [PubMed]
Cite this article as: Fay KA, Finley DJ, Phillips JD. Giant posterior mediastinal schwannoma with ancient features: a case report. Curr Chall Thorac Surg 2020;2:9.