
Flap reconstruction of the chest wall after oncologic resection
Introduction
Infection, oncologic resection, trauma, congenital deformities or radiation necrosis may result in chest wall defects that require reconstruction (1,2). Oncological tumor resection to remove soft tissue sarcoma, osteo or chondrosarcoma, or advanced breast and lung cancer, typically results in severe defects of the chest wall (3,4). These defects are usually classified according to anatomical location including anterior (sternal), anterior-lateral, posterior-lateral and thoraco-abdominal (5).
Thickness of chest wall defects can be classified as full or partial. Partial-thickness defects include defects of the soft tissue and skeletal bone. In the case of oncological resection, one-stage surgery is recommended. In this procedure, resection and reconstruction are conducted in the same operation. In this article, we focus on soft tissue reconstruction of the chest wall after oncological resection.
The goals of chest wall reconstruction
The goals of chest wall reconstruction are generally well-defined. Reconstruction should avoid lung herniation, achieve adequate stability to allow physiological movements, create a stable platform to support the shoulders and upper extremities, and achieve an airtight closure (2,6-8). Reconstruction should also maintain adequate respiratory function, obliterate dead space in the chest wall cavity, and protect the vital intrathoracic organs (9-11).
Coverage with well-vascularized soft tissue is essential not only for achieving the goals of reconstruction (1,7,12), but also for providing an acceptable cosmetic result (2). However, there is still controversy regarding what the size and location of the defect of the chest wall cage should be to necessitate chest wall stabilization (7).
General principles of chest wall reconstruction
Extensive chest wall resection and reconstruction are surgically challenging, but also potentially critical for the survival of the patient. For this reason, careful patient selection is important. A multidisciplinary approach in patient selection, in addition to careful perioperative and post-operative therapy, is essential to achieve the best and earliest possible recovery. The timing and treatment should be individually determined. Surgical operation should be carefully planned in order to achieve a fast and safe operation.
Whether chest wall cage restoration is mandatory for stabilization remains contentious, and largely depends on the size and location of the defect of the chest wall. It has been shown that in larger chest wall defects, reconstruction with mesh has reduced ventilator dependence and hospital stay compared to defects reconstructed without mesh (13).
The size of the chest wall defect is not the only determinant for the need of stabilization; location of the defect is also an important factor. Since the scapula bone provides support for the posterior chest wall, stabilization of the posterior chest wall is required less often (12,14). As such, Mansour et al. suggested that only soft tissue and not skeletal reconstruction would be needed for posterior chest wall defects under the scapula above the fourth rib (4).
In small, full-thickness defects (one or two ribs), some surgeons use synthetic mesh to prevent bulging or herniation of the lung (3,4). It is widely thought that defects over four ribs or larger than 5 cm need stabilization (10,11). Stabilization of the chest wall and soft tissue reconstruction are also needed mainly in larger, full-thickness defects. For larger anterior or anterior-lateral defects, several surgeons have recommended additional stabilization with more rigid techniques such as the sandwich technique (methyl-methacrylate between two meshes) (15), rib graft with mesh, and titanium plates (16). For exclusively skeletal bone defects after chest wall stabilization, soft tissue reconstruction is not mandatory if the primary closure is completed with well-vascularized, healthy soft tissue. If the soft tissue is compromised from previous radiation therapy, the flap reconstruction may endure wound healing. In some select cases, a skin graft is possible, but usually it is suboptimal for the reconstruction of the chest wall defect.
General principles of chest wall flap reconstruction
Pedicled myocutaneous flaps are the first choice for soft tissue reconstruction of the chest wall (1). Sometimes these pedicled or local flaps are inadequate in size and dimension, or are unavailable, in which case microvascular reconstruction may be mandatory (17). It is recommended that the reconstructive elevator algorithm should be used in chest wall soft tissue reconstruction (3).
The choice of reconstruction method is based on the location and size of the defect, availability of the local and pedicled options, patient history of previous operations or radiotherapy, and the general condition and prognosis of the patient. Location of the defect is especially relevant not only when assessing the need of chest wall stabilization, but also for soft tissue reconstruction.
During flap selection, it should be verified that harvesting the area of the flap will not affect breathing (18), and that closing the flap harvesting area will not increase the defect size in the reconstruction area.
Pedicled or local flaps
Pedicled muscle or musculocutaneous flaps
Latissimus dorsi muscle flap
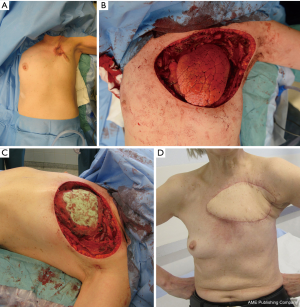
The latissimus dorsi muscle or latissimus dorsi musculocutaneous flap (Figure 1A,B,C,D) has been used as a workhorse for chest wall reconstruction in several surgical series (1,3,4,14,19). This flap is also commonly used to obliterate dead space in intrathoracic defects (20,21), as muscle should not be cut in routine thoracotomy. The latissimus dorsi flap has many advantages, including its large size, its ability to be tailored to the defect, a relatively long pedicle that allows a wide arc of rotation, and its ability to be easily harvested. The skin island of the cutaneous flap can only be harvested to a width of 7–10 cm if the aim is to close the donor site directly. If a large skin island is needed, a donor area has to be skin grafted. The flap can cover most anterior, anterior-lateral, and posterior-lateral defects.
Pectoralis major muscle flap
The pectoralis major muscle flap is another popular choice in chest wall reconstruction (1,14,22). Vascularity of the flap is supplied by a dominant thoracoacromial pedicle and secondary intramammary pedicles. The flap can be used as a pedicle flap based on the thoracoacromial pedicle, or split turnover flap based on secondary pedicles. The flap can be harvested with or without a skin paddle, and can reach to the anterior chest wall. In wider defects, bilateral flaps can be used.
Rectus abdominis muscle flap and variants
A pedicled rectus abdominis muscle flap (19,23) or different musculocutaneous variants (vertical rectus abdominis muscle, VRAM; transversal rectus abdominis, TRAM) have been used to cover anterior, anterior-lateral chest, and thoraco-abdominal wall defects. The rectus abdominis muscle is supplied by two dominant pedicles (the superior epigastric and the deep inferior epigastric artery). For reconstruction of chest wall defects, a pedicled flap is based on the superior pedicle. A VRAM flap design is very suitable for long vertical anterior defects, whereas a TRAM flap can be harvested for a wider, but less reliable, skin paddle without any primary closing problems; ultimately, this resembles an aesthetic abdominoplasty. Harvesting the rectus abdominis muscle is associated with donor morbidity, including the risk of abdominal wall hernia, and could affect early post-operative dynamics of breathing. Ablation or ligation of the ipsilateral internal mammary vessels does not preclude using the rectus abdominis muscle flap. The superior epigastric vascular pedicle can still be perfused through the lower intercostal and musculophrenic artery (24). Musculocutaneous rectus abdominis flaps are particularly likely to develop venous congestion after ligation of the deep inferior epigastric pedicle. In these cases, the flap can be supercharged by vein anastomosis (25).
External oblique muscle
The external oblique muscle flap has also been used in chest wall reconstruction, although the use has not been generalized as in the case of the pectoralis major, rectus abdominis, or latissimus dorsi muscle flaps. Several surgeons (1,26) used this flap in their reconstructions in the nineteenth century, and more recently, Chang et al. (19) have continued to use it. Lee et al. (27) recently reported the results from 75 reconstructions following advanced or recurrent breast cancer. According to their report, complication rates were low, and operation times were under two hours. The flap can be used in anterior-lateral and thoraco-abdominal reconstruction.
Serratus anterior muscle flap
The serratus anterior muscle can be used alone (28) in anterior-lateral and posterior-lateral chest wall reconstruction. The subscapular vascular system also supplies scapular and parascapular flaps. These flaps, including the serratus anterior flap, have been frequently used as chimeric flaps with the latissimus dorsi to repair massive chest wall defects (7).
Pedicled perforator flaps
Thoracodorsal artery perforator (TDAP) flap
The TDAP flap and intercostal artery perforator (ICAP) flap are the most commonly used perforator flaps for chest wall reconstruction. Additional perforator flaps have also been reported in the literature for use in chest reconstruction, including the internal mammary artery perforator, superior epigastric artery perforator (SEAP), lateral thoracic artery perforator, and dorsal scapular artery perforator flaps (29). The thoracoepigastric flap is actually a transposition flap modification of the perforator flap, because usually it is based on the perforator from the epigastric arcade or intercostal arteries. Thoracoepigastric flaps are used to cover smaller defects in the thoracoabdominal area or the lower part of the chest wall (11).
TDAP flaps can cover moderately sized defects on anterior-lateral, posterior-lateral, and anterior chest wall defects. Yang et al. (30) reported 100% flap survival (size 6–9×14–18 cm) with reduced donor site morbidity.
ICAP flap
The ICAP flap can be supplied by anterior, lateral, or posterior ICAPs. Jiang et al. (31) have successfully used a lateral ICAP flap to cover axilla area defects due to lymphatic malformations. Similarly, Yu et al. (32) reported using an anterior ICAP flap to reconstruct a chest wall defect after dermatofibrosarcoma resection.
SEAP flap
The SEAP flap has been used in reconstruction of sternal wound infection after median sternotomy (33,34). The advantages of the SEAP flap include the relatively short operation time (33) and sparing of muscle tissue (34).
Others
The omentum flap
The omentum flap is an alternative option for anterior and anterio-lateral chest wall defect reconstruction (35). The flap can be harvested from traditional open laparotomy (36) or laparoscopy (37). The omentum flap is supplied from unilateral or bilateral gastroepiploic vessels. This flap offers several advantages, including a large surface area, pliability, and a long pedicle. However, the flap has to be covered with a skin graft or another flap, and carries the risk of subsequent hernia.
Breast flap
In special circumstances, a midline chest wall defect can be covered with a breast flap. The major advantage of this flap is fast operation time, particularly in the highly morbid elderly patient cohort (2).
Reverse abdominoplasty
In selected patients, the reverse abdominoplasty has been used successfully in reconstruction of thoraco-abdominal or caudal chest wall defects (38,39). Notably, Pantelides et al. (39) do not advocate reverse abdominoplasty as a first choice for reconstruction. Rather, this should only be an alternative to pedicled or free-flap reconstruction in special cases.
Microvascular free flaps
The indications for microvascular free flaps are the following:
- Pedicle flap options have been used, or the pedicle of these flaps is damaged due to previous operation or radiotherapy (11).
- Some areas are difficult to reach with pedicle flaps, such as thoraco-abdominal defects and defects of the epigastrium area (2).
- A single pedicle flap is not of adequate size or volume to cover the whole defect (10).
General principles of chest wall free-flap reconstruction
In general, the ideal microsurgery flap has a constant anatomy a reliable and sufficiently large pedicle, and the ability to be rapidly harvested. The same principles are important in choosing a microvascular flap for chest wall reconstruction (17).
Microvascular free-flap reconstruction should be carefully planned to achieve a fast and safe operation (2). When possible, a two-team approach is used, and the patient is kept in one position throughout the operation, to avoid a prolonged operation time (3,18).
Usually, recipient vessels are chosen close to the resection site, where healthy vessels are easily accessible. The selection of recipient vessels in two different series is shown in Table 1. Sometimes, these typical recipient vessels are unavailable or the flap positioning is extremely impractical. In these cases, an arterio-venous (A-V) loop is a good option to get inflow and outflow to the flap. Engel et al. (40) created an A-V loop between the cephalic vein and the thoraco-acromial artery (CTA-loop) and used these as recipient vessels. In the thoraco-abdominal region, the saphenous loop from the lower leg is useful to achieve good blood flow to the flap and to relieve positioning of the flap (17).
Table 1
| Recipient vessel | Cordeiro et al. 2001 (25), n=25 | Salo and Tukiainen 2018 (3), n=17 |
|---|---|---|
| Artery | ||
| Internal thoracic artery | 7 | 4 |
| Subclavian artery | 3 | 4 |
| Saphenous loop | – | 3 |
| Subscapular artery | 1 | 3 |
| Thoracodorsal artery | 2 | 2 |
| Axillary artery | 1 | 1 |
| Thoracoacromial artery | 1 | – |
| Transverse cervical artery | 4 | – |
| External carotid artery | 4 | – |
| Lingual artery | 1 | – |
| Brachial | 1 | – |
| Vein | ||
| Subclavian vein | – | 5 |
| Axillary vein | 4 | 3 |
| Saphenous loop | – | 3 |
| Internal thoracic vein | 4 | 2 |
| External jugular vein | 6 | 2 |
| Circumflex scapular vein | – | 1 |
| Thoracodorsal vein | 1 | 1 |
| Innominate vein | 6 | – |
| Internal jugular vein | 4 | – |
| External carotid vein | 4 | – |
| Brachial vein | 1 | – |
In larger chest wall reconstruction series, free-flap reconstruction was necessary in 21% (3), 11% (4), 10% (12), and 6% (19) of the cases. This may reflect patient material and selection. The median chest wall defect size in these series was only reported by Salo et al. (3), and it was 156 m2.
Microvascular free flaps for chest wall reconstruction
Tensor fascia lata (TFL) and variants
Free flaps from thigh areas are very useful and have no negative effect on breathing. These flaps allow flap harvesting and reconstruction in the same position. TFL flap (Figure 2A,B,C,D) is a strong option, as it provides constant anatomy, large flap (up to 20×35 cm), and large vessels to permit safe anastomosis. Usually, the donor side of the TFL flap has to be skin-grafted, and leaves few aesthetic donor deformities.
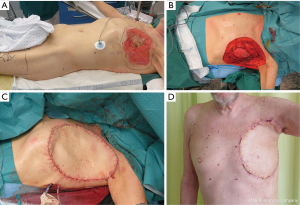
When a particularly large TFL flap is needed (>25×35 cm), including muscle (rectus femoris or vastus lateralis) with the flap gives more perforator vessels to the distal and medial part of the large flap (2,3).
Anterolateral thigh (ALT) flap
The ALT became a popular free soft tissue reconstruction method in the twentieth century (41). The usage of this flap in chest wall reconstruction has increased (18,42,43). A skin paddle can be harvested 8×25 cm (44) with direct closure, and the flap has a long pedicle (42).
Latissimus dorsi flap
Repeated lateral thoracotomies can damage thoracodorsal vessels, rendering the ipsilateral latissimus dorsi muscle flap unusable. The contralateral latissimus dorsi muscle has been successfully used as a free flap (25); in this situation, the position of the patient may need to be changed during the operation. The flap is reliable because of the large diameter of supplying thoracodorsal vessels (2–4 mm). Moreover, donor site morbidity is minimal, and the flap has a large volume. In massive defects, other parascapular flaps can be used in addition to the latissimus dorsi flap.
Rectus abdominis and musculocutaneous alternatives for the rectus abdominis (TRAM and VRAM)
The musculus rectus abdominis flap used to be popular in chest wall reconstruction as either a pedicled (45) or free flap (25), both of which have poor rates for donor site morbidity. After harvesting, there is a defect in the anterior rectus sheath. This can cause a hernia, even when synthetic mesh is used in the reconstruction of the abdominal wall (44).
Presently, musculocutaneous variants of rectus abdominis muscle flap including TRAM, muscle-sparing TRAM, and VRAM, are the microvascular flaps most commonly used in locally advanced breast cancer (43). The flap is reliable, large (2), easy, and can be harvested quickly.
Deep inferior epigastric perforator flap (DIEP)
The DIEP flap has been used for many years as the gold standard in breast reconstruction; hence, surgeons that are specialized in breast reconstruction have extensive experience with the DIEP flap, and the flap is also used in chest wall reconstruction. Donor site morbidity is minimal when the functionality of the rectus muscle is preserved (18). The DIEP flap can cover a large surface area, but closing the harvesting area may increase the anterior chest wall defect (2). Furthermore, the DIEP flap may increase operation time due to intramuscular dissection of the pedicle (44).
Fillet forearm flap
The fillet forearm flap has been used for reconstruction of the chest wall after extended forequarter amputation (46). If the distal part of the arm or/and forearm are tumor-free, fillet forearm flap reconstruction can provide excellent coverage of the extended four quarter area, and also provide contour of the shoulder (2).
Omentum flap
Omentum flap as a free flap is mainly used as a salvage procedure (44).
Survival, complications, and health-related quality of life
Thirty-day mortality in chest wall reconstruction reports ranges from 0 to 7%, which is considered to be acceptable (3,4,11,12,14).
According to the reports on 5-year survival following oncologic chest wall resection and reconstruction, there is wide variation across the different types of cancer; breast cancer ranges from 9% to 69% (3,47-49), soft tissue sarcomas from 55% to 89% (50-53), chondrosarcomas and bone sarcomas from 64% to 92% (3,54,55), and advanced lung cancer from 21% to 61% (56,57). In general, the survival rates of the patients tend to reflect the varying biological behaviors of the different cancers.
At present, surgical complications should be ranked according to the Clavien-Dindo classification of surgical complications. This classification has standardized the estimation of surgical complication in different articles (58). However, only one article regarding reconstruction of the chest wall used this classification to estimate the complications. Therefore, it remains challenging to compare complications among different reports, since different classification systems are used.
Recently, health-related quality of life research (HRQOL) has been recognized as an important part of cancer research, and has become an essential end-point of therapy (59). A recent article was published that evaluated the long-term HRQOL in patients after chest wall reconstruction following oncological resection. According to this publication, the outcome was largely comparable to that of the age- and gender-standardized general population (60), although a slight limitation in breathing or subjective assessment of dyspnoea was noted (60,61).
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Puma and Hon Chi Suen) for the series “Surgical Management of Chest Wall Tumors” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts.2019.12.05/coif). The series “Surgical Management of Chest Wall Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that question related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Arnold PG, Pairolero PC. Chest-wall reconstruction: an account of 500 consecutive patients. Plast Reconstr Surg 1996;98:804-10. [Crossref] [PubMed]
- Tukiainen E. Chest wall reconstruction after oncological resections. Scand J Surg 2013;102:9-13. [Crossref] [PubMed]
- Salo JTK, Tukiainen EJ. Oncologic Resection and Reconstruction of the Chest Wall: A 19-Year Experience in a Single Center. Plast Reconstr Surg 2018;142:536-47. [Crossref] [PubMed]
- Mansour KA, Thourani VH, Losken A, et al. Chest wall resections and reconstruction: a 25-year experience. Ann Thorac Surg 2002;73:1720-5; discussion 1725-6.
- Kuwahara H, Salo J, Tukiainen E. Diaphragm reconstruction combined with thoraco-abdominal wall reconstruction after tumor resection. J Plast Surg Hand Surg 2018;52:172-7. [Crossref] [PubMed]
- Mahabir RC, Butler CE. Stabilization of the chest wall: autologous and alloplastic reconstructions. Semin Plast Surg 2011;25:34-42. [Crossref] [PubMed]
- Althubaiti G, Butler CE. Abdominal wall and chest wall reconstruction. Plast Reconstr Surg 2014;133:688e-701e. [Crossref] [PubMed]
- Thomas PA, Brouchet L. Prosthetic reconstruction of the chest wall. Thorac Surg Clin 2010;20:551-8. [Crossref] [PubMed]
- Bakri K, Mardini S, Evans KK, et al. Workhorse flaps in chest wall reconstruction: the pectoralis major, latissimus dorsi, and rectus abdominis flaps. Semin Plast Surg 2011;25:43-54. [Crossref] [PubMed]
- Netscher DT, Baumholtz MA. Chest reconstruction: I. Anterior and anterolateral chest wall and wounds affecting respiratory function. Plast Reconstr Surg 2009;124:240e-52e. [Crossref] [PubMed]
- Harati K, Kolbenschlag J, Behr B, et al. Thoracic Wall Reconstruction after Tumor Resection. Front Oncol 2015;5:247. [Crossref] [PubMed]
- Losken A, Thourani VH, Carlson GW, et al. A reconstructive algorithm for plastic surgery following extensive chest wall resection. Br J Plast Surg 2004;57:295-302. [Crossref] [PubMed]
- Kroll SS, Walsh G, Ryan B, et al. Risks and benefits of using marlex mesh in chest wall reconstruction. Ann Plast Surg 1993;31:303-6. [Crossref] [PubMed]
- Deschamps C, Tirnaksiz BM, Darbandi R, et al. Early and long-term results of prosthetic chest wall reconstruction. J Thorac Cardiovasc Surg 1999;117:588-91; discussion 591-2. [Crossref] [PubMed]
- Lardinois D, Muller M, Furrer M, et al. Functional assessment of chest wall integrity after methylmethacrylate reconstruction. Ann Thorac Surg 2000;69:919-23. [Crossref] [PubMed]
- Berthet JP, Canaud L, D'Annoville T, et al. Titanium plates and Dualmesh: a modern combination for reconstructing very large chest wall defects. Ann Thorac Surg 2011;91:1709-16. [Crossref] [PubMed]
- Tukiainen E, Popov P, Asko-Seljavaara S. Microvascular reconstructions of full-thickness oncological chest wall defects. Ann Surg 2003;238:794-801; discussion 801-2. [Crossref] [PubMed]
- Arya R, Chow WT, Rozen WM, et al. Microsurgical Reconstruction of Large Oncologic Chest Wall Defects for Locally Advanced Breast Cancer or Osteoradionecrosis: A Retrospective Review of 26 Cases over a 5-Year Period. J Reconstr Microsurg 2016;32:121-7. [Crossref] [PubMed]
- Chang RR, Mehrara BJ, Hu QY, et al. Reconstruction of complex oncologic chest wall defects: a 10-year experience. Ann Plast Surg 2004;52:471-9; discussion 479. [Crossref] [PubMed]
- Chen JT, Bonneau LA, Weigel TL, et al. A Twelve-Year Consecutive Case Experience in Thoracic Reconstruction. Plast Reconstr Surg Glob Open 2016;4:e638. [Crossref] [PubMed]
- Arnold PG, Pairolero PC. Intrathoracic muscle flaps: a 10-year experience in the management of life-threatening infections. Plast Reconstr Surg 1989;84:92-8; discussion 99. [Crossref] [PubMed]
- Azoury SC, Grimm JC, Tuffaha SH, et al. Chest Wall Reconstruction: Evolution Over a Decade and Experience With a Novel Technique for Complex Defects. Ann Plast Surg 2016;76:231-7. [Crossref] [PubMed]
- Weyant MJ, Bains MS, Venkatraman E, et al. Results of chest wall resection and reconstruction with and without rigid prosthesis. Ann Thorac Surg 2006;81:279-85. [Crossref] [PubMed]
- Netscher DT, Eladoumikdachi F, Goodman CM. Rectus abdominis muscle flaps used successfully for median sternotomy wounds after ipsilateral internal mammary artery ligation. Ann Plast Surg 2001;47:223-8. [Crossref] [PubMed]
- Cordeiro PG, Santamaria E, Hidalgo D. The role of microsurgery in reconstruction of oncologic chest wall defects. Plast Reconstr Surg 2001;108:1924-30. [Crossref] [PubMed]
- Bogossian N, Chaglassian T, Rosenberg PH, et al. External oblique myocutaneous flap coverage of large chest-wall defects following resection of breast tumors. Plast Reconstr Surg 1996;97:97-103. [Crossref] [PubMed]
- Lee S, Jung Y, Bae Y. Immediate chest wall reconstruction using an external oblique myocutaneous flap for large skin defects after mastectomy in advanced or recurrent breast cancer patients: A single center experience. J Surg Oncol 2018;117:124-9. [Crossref] [PubMed]
- Arnold PG, Pairolero PC, Waldorf JC. The serratus anterior muscle: intrathoracic and extrathoracic utilization. Plast Reconstr Surg 1984;73:240-8. [Crossref] [PubMed]
- Florczak AS, Chaput B, Herlin C, et al. The Use of Pedicled Perforator Flaps in Chest Reconstruction: A Systematic Review of Outcomes and Reliability. Ann Plast Surg 2018;81:487-94. [Crossref] [PubMed]
- Yang LC, Wang XC, Bentz ML, et al. Clinical application of the thoracodorsal artery perforator flaps. J Plast Reconstr Aesthet Surg 2013;66:193-200. [Crossref] [PubMed]
- Jiang Z, Li S, Kretlow JD, et al. Closure of large defects after microcystic lymphatic malformations using lateral intercostal artery perforator flap. J Plast Reconstr Aesthet Surg 2014;67:1230-6. [Crossref] [PubMed]
- Yu S, Zang M, Xu L, et al. Perforator Propeller Flap for Oncologic Reconstruction of Soft Tissue Defects in Trunk and Extremities. Ann Plast Surg 2016;77:456-63. [Crossref] [PubMed]
- Wettstein R, Weisser M, Schaefer DJ, et al. Superior epigastric artery perforator flap for sternal osteomyelitis defect reconstruction. J Plast Reconstr Aesthet Surg 2014;67:634-9. [Crossref] [PubMed]
- Eburdery H, Grolleau JL, Berthier C, et al. Management of Large Sternal Wound Infections With the Superior Epigastric Artery Perforator Flap. Ann Thorac Surg 2016;101:375-7. [Crossref] [PubMed]
- Hultman CS, Culbertson JH, Jones GE, et al. Thoracic reconstruction with the omentum: indications, complications, and results. Ann Plast Surg 2001;46:242-9. [Crossref] [PubMed]
- Jurkiewicz MJ, Arnold PG. The omentum: an account of its use in the reconstruction of the chest wall. Ann Surg 1977;185:548-54. [Crossref] [PubMed]
- Pechetov AA, Esakov YS, Makov MA, et al. Laparoscopic-assisted harvesting of omental flap in chest wall reconstruction for deep sternal wound infection. Khirurgiia (Mosk) 2017;18-23. [Crossref] [PubMed]
- Bury TF, Reece GP, Janjan NA, et al. Closure of massive chest wall defects after full-thickness chest wall resection. Ann Plast Surg 1995;34:409-14. [Crossref] [PubMed]
- Pantelides NM, Mondal D, Wishart GC, et al. Reverse abdominoplasty: a practical option for oncological trunk reconstruction. Eplasty 2013;13:e2. [PubMed]
- Engel H, Pelzer M, Sauerbier M, et al. An innovative treatment concept for free flap reconstruction of complex central chest wall defects--the cephalic-thoraco-acromial (CTA) loop. Microsurgery 2007;27:481-6. [Crossref] [PubMed]
- Wei FC, Jain V, Celik N, et al. Have we found an ideal soft-tissue flap? An experience with 672 anterolateral thigh flaps. Plast Reconstr Surg 2002;109:2219-26; discussion 2227-30. [Crossref] [PubMed]
- Di Candia M, Wells FC, Malata CM. Anterolateral thigh free flap for complex composite central chest wall defect reconstruction with extrathoracic microvascular anastomoses. Plast Reconstr Surg 2010;126:1581-8. [Crossref] [PubMed]
- Song D, Liu D, Pafitanis G, et al. Extensive Microsurgical Reconstruction of Chest Wall Defects for Locally Advanced Breast Cancer: A 10-Year Single-Unit Experience. Ann Plast Surg 2019; [Epub ahead of print]. [Crossref] [PubMed]
- Sauerbier M, Dittler S, Kreutzer C. Microsurgical chest wall reconstruction after oncologic resections. Semin Plast Surg 2011;25:60-9. [Crossref] [PubMed]
- Neale HW, Kreilein JG, Schreiber JT, et al. Complete sternectomy for chronic osteomyelitis with reconstruction using a rectus abdominis myocutaneous island flap. Ann Plast Surg 1981;6:305-14. [Crossref] [PubMed]
- Cordeiro PG, Cohen S, Burt M, et al. The total volar forearm musculocutaneous free flap for reconstruction of extended forequarter amputations. Ann Plast Surg 1998;40:388-96. [Crossref] [PubMed]
- Daigeler A, Druecke D, Hakimi M, et al. Reconstruction of the thoracic wall-long-term follow-up including pulmonary function tests. Langenbecks Arch Surg 2009;394:705-15. [Crossref] [PubMed]
- Petrella F, Radice D, Borri A, et al. Chest wall resection and reconstruction for locally recurrent breast cancer: From technical aspects to biological assessment. Surgeon 2016;14:26-32. [Crossref] [PubMed]
- Levy Faber D, Fadel E, Kolb F, et al. Outcome of full-thickness chest wall resection for isolated breast cancer recurrence. Eur J Cardiothorac Surg 2013;44:637-42. [Crossref] [PubMed]
- Kuwahara H, Salo J, Nevala R, et al. Single-Institution, Multidisciplinary Experience of Soft Tissue Sarcomas in the Chest Wall. Ann Plast Surg 2019;83:82-8. [Crossref] [PubMed]
- Gross JL, Younes RN, Haddad FJ, et al. Soft-tissue sarcomas of the chest wall: prognostic factors. Chest 2005;127:902-8. [Crossref] [PubMed]
- Pfannschmidt J, Geisbusch P, Muley T, et al. Surgical treatment of primary soft tissue sarcomas involving the chest: experiences in 25 patients. Thorac Cardiovasc Surg 2006;54:182-7. [Crossref] [PubMed]
- Tsukushi S, Nishida Y, Sugiura H, et al. Soft tissue sarcomas of the chest wall. J Thorac Oncol 2009;4:834-7. [Crossref] [PubMed]
- Soerensen TR, Raedkjaer M, Jorgensen PH, et al. Soft Tissue Sarcomas of the Thoracic Wall: More Prone to Higher Mortality, and Local Recurrence-A Single Institution Long-Term Follow-up Study. Int J Surg Oncol 2019;2019:2350157. [Crossref] [PubMed]
- Burt M, Fulton M, Wessner-Dunlap S, et al. Primary bony and cartilaginous sarcomas of chest wall: results of therapy. Ann Thorac Surg 1992;54:226-32. [Crossref] [PubMed]
- Facciolo F, Cardillo G, Lopergolo M, et al. Chest wall invasion in non-small cell lung carcinoma: a rationale for en bloc resection. J Thorac Cardiovasc Surg 2001;121:649-56. [Crossref] [PubMed]
- Magdeleinat P, Alifano M, Benbrahem C, et al. Surgical treatment of lung cancer invading the chest wall: results and prognostic factors. Ann Thorac Surg 2001;71:1094-9. [Crossref] [PubMed]
- Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205-13. [Crossref] [PubMed]
- Bottomley A, Aaronson NKEuropean Organisation for Research and Treatment of Cancer. International perspective on health-related quality-of-life research in cancer clinical trials: the European Organisation for Research and Treatment of Cancer experience. J Clin Oncol 2007;25:5082-6. [Crossref] [PubMed]
- Salo JTK, Repo JP, Roine RP, et al. Health-related quality of life after oncological resection and reconstruction of the chest wall. J Plast Reconstr Aesthet Surg 2019;72:1776-84. [Crossref] [PubMed]
- Heuker D, Lengele B, Delecluse V, et al. Subjective and objective assessment of quality of life after chest wall resection. Eur J Cardiothorac Surg 2011;39:102-8. [Crossref] [PubMed]
Cite this article as: Salo J, Tukiainen E. Flap reconstruction of the chest wall after oncologic resection. Curr Chall Thorac Surg 2020;2:5.


