
Thoracoscopic lobectomy for lung cancer in challenging cases: technical aspects
Introduction
From some years, the National Comprehensive Cancer Network (NCCN) practice guidelines for non-small cell lung cancer (NSCLC) recommend video-assisted thoracoscopic surgery (VATS) and the respect of the standard oncologic and dissection principles remains a sine qua non condition for the minimally invasive thoracic surgery (1). Several reports have shown that VATS lobectomy is safe for resectable NSCLC, emphasizing exciting outcomes of this procedure when compared with conventional thoracotomy as shorter length of hospital stay, more rapid return to normal activities and higher postoperative quality of life (2,3). The accumulation of experience together with concurrent improvements in instrumentation and video-technology have enabled the evolution of VATS lobectomy in the management of more complex cases and in high-volume centers, where there is significant expertise with minimally invasive thoracic procedures, extensive adhesions obliterating the pleural space, incompleteness of interlobar fissures, preoperative chemo-radiotherapy, previous thoracic surgery, chest wall involvement or centrally located tumors are considered a relative contraindications (4,5).
T factor in NSCLC
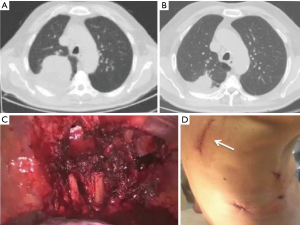
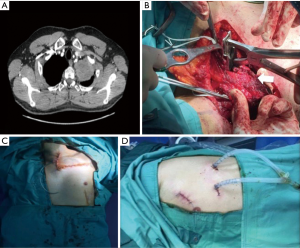
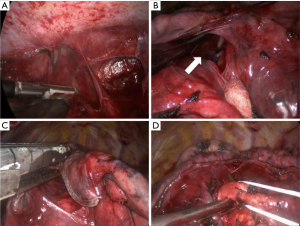
NSCLC with chest wall involvement, after careful nodal and systemic staging, can be treated by VATS and in thoracic surgical units the non-availability of endoscopic rib-cutting instruments is compensated by using, in selected cases, a conventional rib cutter through a hybrid approach: the procedure involves a thoracoscopic lobectomy combined with a limited incision centred directly over the area of planned chest wall resection, with no rib spreading and scapular mobilization (Figure 1). However, patients requiring resection of 4 or more ribs, those with transverse processes involvement or with area of chest wall too close to the incisions aren’t optimal candidates for this approach (6). Our experience suggests that a hybrid approach can be useful even in patients with NSCLC invading the first rib in the anterior part of the superior sulcus: in such instance a thoracoscopic lobectomy is combined with an anterior transmanubrial approach for en-bloc chest wall resection through the elevation of an osteomuscolar flap formed by sternocleidomastoid muscle, clavicle, first rib and cut sternal manubrium, as described in 1997 (7) (Figure 2). With increased experience in minimally invasive surgery, even the tumor size becomes a relative contraindication to VATS lobectomy: a careful hilar dissection and lung mobilization can avoid the risk of vascular injury due to excessive traction on small arterial branches or incorrect identification of appropriate tissue plans that are usually thicker and more adherent after neoadjuvant chemotherapy. Advanced thoracoscopic skills are need especially for centrally located large tumors in close proximity to major vascular structures where preservation of lung tissue by means of bronchoplastic procedures and vascular sleeve resection is highly recommended in patients at increased risk for complications after pneumonectomy (8). Also the treatment of early stage NSCLC sometimes requires technically demanding procedures as in patients undergoing lobectomy or completion pneumonectomy by VATS after prior lung resection, due to perihilar fibrosis or diffuse pleural adhesions: in this situation, isolation of vessels can be difficult and an opening of the pericardium may be necessary if the hilar adhesions are hard enough to dissect extrapericardially (9). Moreover, in presence of dense fissure, to avoid air leaks following lobectomy in redo VATS, we recommend the so-called tunnel technique (10): this type of parenchymal dissection allows a complete interlobar lymph node evaluation and provides an optimal view of the bronchovascular structures (Figure 3). Early stage NSCLC treatment can be challenging in other conditions as in patients with previous coronary artery bypass using left internal mammary artery (LIMA) or right internal mammary artery (RIMA) through median sternotomy: in these patients, during pleural adhesions lyse, cautious attention to avoid damages of LIMA or RIMA grafts is mandatory.
N1 disease in NSCLC
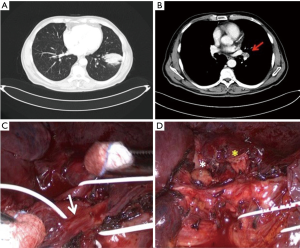
Systematic lymphadenectomy is the standard procedure in the surgical treatment for NSCLC and allows adequate pathological staging. However, in patients with pathologic N1 disease surgical management can be complex in presence of hilar bulky lymphadenopathy: this condition often requires the incision of the vascular sheath with dissection of blood vessels within the sheath and a meticulous combination of sharp and blunt dissection when the fissure is separated to avoid unintentional injuries to segmental arteries or anomalous venous branches (11), due to enlarged lymph nodes that obscure local anatomic structures. Metastases in hilar lymph nodes, fused and closely adherent to the bronchovascular structures, make minimally invasive surgery more difficult to perform and can lead to intraoperative bleeding from arterial injury. This circumstance is one of the main reasons for planned conversion or urgent conversion to open thoracotomy during VATS lobectomy (12). VATS surgeons with advanced technical skills may be more able, than surgeons with low-level of experience in minimally invasive techniques, to prevent hemorrhagic complications during dissection of malignant lymph nodes infiltrating blood vessels, performing a proximal and distal control of the pulmonary artery followed by partial pulmonary arterioplasty or bronchovascular sleeve resection (13,14). In selected cases of left lower lobe cancer and metastatic interlobar lymph nodes with extracapsular spread, to achieve a complete lymph node dissection during VATS lobectomy, it is possible to perform resection of lower lobe with partial preservation of segmental arteries of left upper lobe, as we have recently reported (15) (Figure 4). In patients with left lower lobe lung cancer and bulky N1 disease or pathologic interlobar lymph nodes adherent to the bronchus, other surgical procedures can be adopted as pneumonectomy (16) or lingular segmentectomy and left lower lobectomy via unique bronchial dissection (17).
Conclusions
For challenging cases, the treatment of resectable NSCLC by minimally invasive technique should be restricted to high-volume centers with significant VATS experience. Centrally located lung cancers and extracapsular spread of hilar lymph node metastases require specific skills during VATS lobectomy for prevention and management of intraoperative complications. Whenever possible, thoracoscopic lung tissue-sparing anatomical resections are strongly advisable.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2.2013. J Natl Compr Canc Netw 2013;11:645-53. [Crossref] [PubMed]
- Villamizar NR, Darrabie MD, Burfeind WR, et al. Thoracoscopic lobectomy is associated with lower morbidity compared with thoracotomy. J Thorac Cardiovasc Surg 2009;138:419-25. [Crossref] [PubMed]
- Paul S, Altorki NK, Sheng S, et al. Thoracoscopic lobectomy is associated with lower morbidity than open lobectomy: a propensity-matched analysis from the STS database. J Thorac Cardiovasc Surg 2010;139:366-78. [Crossref] [PubMed]
- Pischik VG. Technical difficulties and extending the indications for VATS lobectomy. J Thorac Dis 2014;6:S623-30. [PubMed]
- Hanna JM, Berry MF, D’ Amico TA. Contraindications of video-assisted thoracoscopic surgical lobectomy and determinants of conversion to open. J Thorac Dis 2013;5:S182-9. [PubMed]
- Berry MF, Onaitis MW, Tong BC, et al. Feasibility of hybrid thoracoscopic lobectomy and en-bloc chest wall resection. Eur J Cardiothorac Surg 2012;41:888-92. [Crossref] [PubMed]
- Grunenwald D, Spaggiari L. Transmanubrial osteomuscolar sparing approach for apical chest tumors. Ann Thorac Surg 1997;63:563-6. [Crossref] [PubMed]
- Soultanis KM, Chen Chao M, Chen J, et al. Technique and outcomes of 79 consecutive uniportal video-assisted sleeve lobectomies. Eur J Cardiothorac Surg 2019;56:876-82. [Crossref] [PubMed]
- Tabutin M, Couraud S, Guibert B, et al. Completion pneumonectomy in patients with cancer: postoperative survival and mortality factors. J Thorac Oncol 2012;7:1556-62. [Crossref] [PubMed]
- Decaluwe H, Sokolow Y, Deryck F, et al. Thoracoscopic tunnel technique for anatomical lung resections: a ‘fissure first, hilum last’ approach with staplers in the fissureless patient. Interact Cardiovasc Thorac Surg 2015;21:2-7. [Crossref] [PubMed]
- Amore D, Casazza D, Imitazione P, et al. Common and uncommon variations of pulmonary venous drainage in patients undergoing thoracoscopic lobectomy. Thorac Cardiovasc Surg 2019. [Epub ahead of print]. [PubMed]
- Li Y, Wang J. Analysis of lymph node impact on conversion of complete thoracoscopic lobectomy to open thoracotomy. Thorac Cancer 2015;6:704-8. [Crossref] [PubMed]
- Gonzalez-Rivas D, Fieira E, Delgado M, et al. Is uniportal thoracoscopic surgery a feasible approach for advanced stages of non-small cell lung cancer? J Thorac Dis 2014;6:641-8. [PubMed]
- Fan J, Yao J, Wang Q, et al. Safety and feasibility of uniportal video-assisted thoracoscopic surgery for locally advanced non-small cell lung cancer. J Thorac Dis 2016;8:3543-50. [Crossref] [PubMed]
- Amore D, Casazza D, Bergaminelli C, et al. Left lower lobectomy and partial preservation of segmental arteries of left upper lobe: A strategy to avoid pneumonectomy in selected cases. Thoracic Cancer 2019;10:1837-40. [Crossref] [PubMed]
- Samejima J, Nakao M, Matsuura Y, et al. Prognostic impact of bulky swollen lymph nodes in cN1 non-small cell lung cancer patients. Jpn J Clin Oncol 2015;45:1050-4. [Crossref] [PubMed]
- Higuchi R, Nakagomi T, Shikata D, et al. Lingular segmentectomy and left lower lobectomy via unique bronchial dissection. J Thorac Dis 2018;10:E420-5. [Crossref] [PubMed]
Cite this article as: Curcio C, Amore D, Casazza D, Saglia A, Imitazione P, Izzo A, Rispoli M, Nespoli MR. Thoracoscopic lobectomy for lung cancer in challenging cases: technical aspects. Curr Chall Thorac Surg 2019;1:25.


