
Management of total esophageal obstruction after stenting for sleeve gastrectomy leak
Introduction
Leaks after sleeve gastrectomy occur in up to 3% of patients and the management of these complications may be challenging. Endoscopic therapies are increasingly used and include clips, transmural pigtail stents, and fully covered esophageal stents (1,2). Stents are associated with an overall 15–45% rate of migration, while other adverse events such as bleeding, obstruction or perforation are rare (3). Persistent leaks may require a revisional surgical procedure, including laparoscopic drainage, completion gastrectomy and esophagojejunostomy, or, most commonly, a Roux-en-Y fistulo-jejunostomy (4). Interestingly, successful endoscopic therapy can be complicated by total esophageal obstruction due to dense fibrosis at the proximal margin of a fully covered stent.
Case presentation
A 35-year-old woman with body mass index 56 kg/m2 and history of previous open left colectomy for a desmoplastic small round cell tumor, underwent laparoscopic sleeve gastrectomy. The immediate post-operative course was unremarkable and she was discharged home on post-operative day 2. The patient was readmitted on postoperative day 5 with fever, and a CT abdominal scan revealed a splenic infarction that was treated conservatively. She was again re-admitted a few days later for persistent fever. Repeat CT abdominal scan showed a 3-cm in size air-fluid collection in the left hypochondrium and a left pleural effusion. An intra-abdominal abscess was suspected, and an explorative laparoscopy was performed to drain the collection. A leakage from the upper staple line was also identified, and a fully covered endoscopic stent (NITI-S Beta 2, 200 mm × 24 mm, Taewoong Medical Industries, Kangseo-Gu Songjung-Dong, South Korea) was deployed intraoperatively. The procedure was uneventful, and the patient was discharged home a few days later eating a soft diet. The stent was removed two months after the implant. Subsequently, the patient noticed the gradual onset of dysphagia for both solids and liquids, and persistent low-grade fever. An upper gastrointestinal endoscopy was performed one month after stent removal and revealed a severe esophageal stricture at 33 cm from incisors. No endoscopic dilation was attempted, proton pump inhibitors and broad-spectrum antibiotics were administered, and parenteral nutrition through a central venous line was initiated.
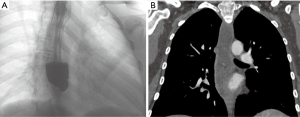
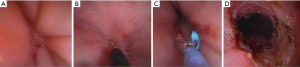
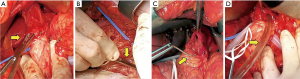
The patient was subsequently referred to our center. She was in stable general conditions but under total parenteral nutrition, still unable to swallow the saliva, and with persistent low-grade fever. A gastrografin swallow study and thoraco-abdominal CT scan confirmed the site of obstruction, about 5 cm above the gastroesophageal junction, and a marked retro-dilatation of the esophagus (Figure 1). Also, a 2 cm intra-splenic abscess was found. An attempt to dilate the fibrotic esophageal obstruction was unsuccessful because no residual lumen was visible. Through a median laparotomy, lysis of visceral adhesions and splenectomy were performed. The esophago-gastric junction was identified and the lower mediastinum was dissected to gain access to the distal esophagus. An intraoperative endoscopy was simultaneously performed to assess the viability of the distal esophagus, but it was impossible to palpate the tip of the endoscope from the hiatus. Through a 1-cm proximal gastrotomy, a rendez-vous maneuver was attempted by introducing and advancing orally a 12 Fr Hegar dilator until a resistance was encountered. While pressing with the tip of the Hegar dilator against the endoluminal resistance, a small incision was made via endoscopy in the midline of the scar area occluding the esophageal lumen with an electrocautery hook, until the tip of the Hegar dilator became visible. Hence, a flexible guidewire was advanced through the stricture and retrieved by the surgeon. Initial balloon dilation of the stenotic tract was performed, followed by progressive dilatations using the series of Savary-Gillard dilators no. 7, 9, and 11 mm. The gastrotomy incision was closed with a barbed suture. A fully covered Ultraflex (Boston Scientific) stent was then deployed for temporary remodeling of the esophageal lumen (Figures 2,3) On postoperative day 5, a gastrografin swallow study showed normal transit of the contrast through the stent. The patient was allowed to eat a soft diet and was discharged on postoperative day 7. The stent was removed 3 weeks later. Repeat endoscopy one and three months after the procedure showed healing of the esophageal mucosa and complete esophageal lumen restoration.
Discussion
To the best of our knowledge, only one case report in the literature has previously described a distal esophageal stricture due to stent placement for a leak occurring after sleeve gastrectomy and subsequent Roux-en-Y fistulo-jejunostomy (5). However, our patient is unique because of an undilatable esophageal obstruction at presentation, and because of the endoscopic and surgically-assisted rendez-vous procedure that allowed prompt lumen recanalization. The combined endoscopic and open surgical approach was justified by the total obliteration of esophageal lumen and by the need to perform a concomitant splenectomy due to the chronic splenic abscess causing persistent fever. The decision to add a temporary full-covered stent after the procedure was made to avoid the trauma of repeated short-term dilation sessions.
Procedure-related adverse events after sleeve gastrectomy can be life-threatening and associated with high morbidity and costs (6), and quite often lead to revisional surgery (4). Leakage of the staple-line suture at the angle of His is the most common and feared complication of sleeve gastrectomy. Results from a large multicenter cohort study have shown that endoscopic therapy of leaks after sleeve gastrectomy is appropriate and can avoid surgical revision in 73% of patients with an acceptable adverse-event profile. However, the most common complication is stent migration that occurs in nearly 25% of patients. There is general consensus that optimal time for stent removal is between 6 and 8 weeks post-implant to prevent complications related to tissue ingrowth and scarring at the proximal end (3).
In conclusion, stenting for treatment of leaks after sleeve gastrectomy can be complicated by overgrowth of scar tissue at the proximal end causing complete esophageal obstruction. This is an extremely rare complication of an otherwise successful stenting procedure. The endoscopic and surgical rendez-vous procedure we described allows safe esophageal lumen restoration via surgically-assisted endoscopic stricturotomy, progressive endoscopic dilatation, and fully covered stenting for temporary remodeling of the esophageal lumen.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ccts.2019.08.04). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Rosenthal RJ. International Sleeve Gastrectomy Expert Panel Consensus Statement: best practice guidelines based on experience of >12,000 cases. Surg Obes Relat Dis 2012;8:8-19. [Crossref] [PubMed]
- Puli SR, Spofford IS, Thompson CC. Use of self-expandable stents in the treatment of bariatric surgery leaks: a systematic review and meta-analysis. Gastrointest Endosc 2012;75:287-93. [Crossref] [PubMed]
- Smith ZL, Park KH, Llano EM, et al. Outcomes of endoscopic treatment of leaks and fistulae after sleeve gastrectomy: results from a large multicenter U.S. cohort. Surg Obes Relat Dis 2019;15:850-5. [Crossref] [PubMed]
- Brethauer SA, Kothari S, Sudan R, et al. Systematic review on reoperative bariatric surgery: American Society for Metabolic and Bariatric Surgery Revision Task Force. Surg Obes Relat Dis 2014;10:952-72. [Crossref] [PubMed]
- Nedelcu M, Manos T, Gagner M, et al. Cost analysis of leak after sleeve gastrectomy. Surg Endosc 2017;31:4446-50. [Crossref] [PubMed]
- Kary N, Chahine E, Moryoussef F, et al. Esophageal Stricture Due to a Self-Expandable Metal Stent (SEMS) Placement for Post Sleeve Gastrectomy Leak: a Case Report. Obes Surg 2019;29:1943-5. [Crossref] [PubMed]
Cite this article as: Milito P, Siboni S, Bernardi D, Asti E, Bonavina L. Management of total esophageal obstruction after stenting for sleeve gastrectomy leak. Curr Chall Thorac Surg 2019;1:11.


