Tracheal surgery: still a challenge or a reality for thoracic surgeons?—A 30-month single-centre experience
Introduction
Tracheal surgery still represents a challenging ground for thoracic surgeons, despite the first tracheal resection was performed by Belsey in 1950 (1). Since then, the development of surgical techniques begun to accelerate (2); however the history of tracheal surgery has been characterized by a long and slow paced due to the peculiar anatomical features of this airway tract. In particular, anatomic location of the trachea, its length, structural rigidity and blood supply need to be always considered when approaching to this organ.
A large variety of indications for tracheal resection and reconstruction (TRR) exist, which can be allocated into two major groups: neoplastic and non-neoplastic diseases.
Neoplastic diseases are classified as follows:
- Primary tracheal tumours (malignant or benign): rare, approximately 0.2% of all tumours of the respiratory tract (3). In adults 90% of all tracheal tumours are malignant (4,5). The two-thirds of tracheal carcinomas are squamous cell carcinoma and adenoid cystic carcinoma, followed by non-squamous bronchogenic carcinoma, mucoepidermoid carcinoma, carcinoid tumours, sarcomas, melanomas, and lymphomas. Benign tumours are found in 11–13% of cases and they are represented by granular cell tumours, fibrous histiocytoma, chondroma, fibroma, hemangioma and papilloma (4,5).
- Secondary malignant tracheal tumours: typically arise from locally spread of neoplasms of laryngeal structures, thyroid gland, esophagus, lung/bronchi, or as metastatic disease (6).
Non-neoplastic diseases are a heterogeneous group including:
- Post-intubation, post-tracheostomy or post-traumatic circumferential tracheal stenosis, which originate from high-pressure endotracheal or tracheostomy cuffs, inflammation, infection and necrosis of the trachea (7,8).
- Idiopathic laryngotracheal stenosis (ILTS): a rare condition characterized by an inflammatory cicatricial stenosis at the level of the cricoid cartilage and proximal trachea, which almost entirely affects younger women (9).
- Congenital and post-infectious lesions represent a rare and wide spectrum of diseases including short- or long-segment stenosis, cartilaginous fibrosis, or calcified nodules (10,11).
- Tracheoesophageal fistulas (TEF) and tracheoinnominate fistulas: rare life-threatening conditions due to long-term high-pressure endotracheal tube and tracheostomy cuffs, or indwelling tracheal or esophageal stents, which cause areas of granulation, malacia, and eventual erosion into surrounding structures (11,12). Fistulas may also be a consequence of caustic ingestion or penetrating trauma to the airway (13).
- Tracheal injuries: most causes include blunt trauma, penetrating trauma, iatrogenic causes (post-intubation and post-tracheotomy), inhalation and aspiration of liquids or objects (14). Regardless of its nature, these lesions may be life-threatening. Despite early recognition and appropriate management, potential complications consist of decreased lung function, infection, vocal cord paralysis and strictures (15).
To date, surgery remains the mainstay of treatment of tracheal diseases.
Taking into account the peculiar anatomical features of the larynx and the trachea, the relative rarity of tracheal diseases and the technical difficulties of surgery of this airway tract, which is burdened by potentially severe complications, a careful and extensive preoperative evaluation of the patient is mandatory. Furthermore, this kind of patients should be managed in high-volume centres, with an experienced multidisciplinary medical team.
In the current paper we reported our preliminary experience in tracheal surgery, particularly evaluating its feasibility, safety and efficacy, through the analysis of short- and long-term outcomes.
Methods
Twenty-two patients affected by ILTS, post-intubation laryngotracheal and tracheal stenosis, tracheal cancer, TEF and post-traumatic tracheal lacerations have been referred to us from February 2017. The prospectively collected clinical data of these patients were retrospectively reviewed. All patients gave full consent regarding data collection and its use in clinical studies; moreover, they gave their consent to update database information.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was evaluated by the Institutional Review Board (IRB) of San Camillo Forlanini Hospital and, as this was a retrospective review for service evaluation (within an audit approved by our Surgical Department) and there was no modification in patients’ care (no prospective randomized study), we did not need the final ethical approval of our IRB.
All patients underwent preoperative evaluation by computed tomographic (CT) imaging, flexible and rigid tracheobronchoscopy to assess the length of diseased trachea, laryngeal involvement, and vocal cord function, as well as presence of secretions, inflammation, laceration or obstruction. Any previously placed stents have been removed via rigid tracheobronchoscopy. Biopsy was performed for any endoluminal lesions. Patients affected by tracheal cancer underwent positron emission tomography (PET-CT) for stadiation of disease. Esophagoscopy was routinely done to complete the assessment of the location, extension and size of TEFs.
Laryngotracheal stenosis was classified according to McCaffrey clinical stages (16) and Cotton-Myer grading system (17).
Morbidity and mortality as well as data on the postoperative hospitalization were recorded.
All the follow-up data (vital status, adverse events after discharge, presence of any tumour or stenosis recurrence) were collected from medical reports and by phone. No patient was lost to follow-up.
Surgical technique
All patients were positioned supine with the neck flexed and posteriorly hyperextended. The surgical access was decided based on disease localization and extent. A collar cervical incision was usually adequate to approach subglottic larynx and cervical trachea. A cervical collar incision combined with partial or total sternotomy was performed in case of middle third tracheal lesions or extensive TEFs to better expose the surgical field.
All patients but post-traumatic lacerations were treated with a single-staged laryngotracheal resection and reconstruction (LTRR) or TRR with primary end-to-end anastomosis. The release techniques consisted in the digitally mobilization of the pretracheal plane to the carena or during video-mediastinoscopy and partial Dedo-Fishman suprathyroid laryngeal release. We usually limited the release to the section of the thyro-hyoid muscles to avoid inhalation complication (18).
Patients with subglottic stenosis underwent a modification of the standard technique of anterior cricoid resection, as described by Pearson and colleagues (19). A Pearson modified subglottic LTRR according to Liberman-Mathisen—tailored cricoplasty—was performed to allow increased postoperative lateral luminal airway diameter (20). In two cases of infraglottic idiopathic recurrent stenosis we performed the Grillo’s procedure consisting in the mucosectomy of the cricoid plate resurfaced with a membranacea mucosal flap (21).
A single-stage TRR with primary esophageal closure has been adopted for TEFs, with or without pedicled tissue flaps interposition (22).
A direct suture of the tracheal defect has been performed for post-traumatic lacerations.
Regarding anesthesiological principles, the airway was intubated under direct visualization distal to the lesion with a flexible single-lumen endotracheal tube. A total intravenous technique with short-acting muscle relaxants, quickly metabolized anaesthetic agents and short-acting opioids has been adopted to let spontaneous ventilation during the operation and early extubation. The endotracheal tube was withdrawn under flexible tracheoscopy to localize the exact disease extension before the airway transection. Once the trachea was divided below the damaged area, ventilation was obtained by a cross-field endotracheal tube into the distal tracheal airway. All laryngotracheal and tracheal anastomosis were performed under apneic hyperoxygenation (preoxygenation with an inspired oxygen fraction of 100%. During airway reconstruction a nasogastric tube was introduced into the distal airway to provide apneic hyperoxygenation via an oxygen flow of 12 to 15 L/min) (23). After the posterior wall anastomosis, the original endotracheal tube was pulled into field distal to the suture line.
Perioperative management
All patients were extubated in the operating room under bronchoscopic view to assess the mobility of vocal cords. They were maintained mild neck flexion for 10 days postoperatively; some patients, especially if they were affected by neurological disorders, had a “guardian stitch” to avoid neck hyperextension. All the patients underwent flexible bronchoscopy before discharge to evaluate the adequacy of the airway and the integrity of suture line. Laryngeal oedema occasionally occurred in post-operative course and it was treated by steroids.
Follow-up examination usually included tracheobronchoscopy controls and CT scans, according to the benignant or malignant nature of patient’s disease. In asymptomatic patients, flexible tracheobronchoscopy was performed at 30 days, 6 months, 12 months, every year for the first 5 years after surgery.
Statistical analysis
Descriptive statistics were used to label the patients included in the study. Continuous variables were expressed as mean and standard deviation. Categorical variables were defined as absolute and relative percentages.
Perioperative mortality included all deaths occurring within 30 days of surgery and those who died later but during the same hospitalization. Overall survival (OS) was estimated with Kaplan-Meier analysis with a 95% confidence interval (CI). The date of the initial operation was the starting point in the survival evaluation and the date of death or last follow-up the endpoint. Univariate analysis was carried out to assess potential risk factors in the development of postoperative complications. Multivariate analysis with the Cox proportional hazards model was applied to identify predictors affecting the OS. Statistical significance was fixed at P value less than 0.05.
Statistical analysis was performed with SPSS software Version 26.0 for Windows (SPSS, IBM, Chicago, IL, USA).
Results
Twenty-two patients underwent tracheal surgery during a 30-month period. The main clinical characteristics of the population are summarized in Table 1. There were 14 (63.6%) male and 8 (36.4%) female. The mean age of the population was 54 (range 21–83) years. Thirteen (59.1%) patients presented a laryngotracheal involvement, while 9 (40.9%) patients had a tracheal disease localization. Nineteen (86.4%) patients underwent LTRR or TRR. Nine (40.9%) resections were performed for post-intubation laryngotracheal/tracheal stenosis, 4 (18.2%) resections for TEFs, 4 (18.2%) resections were performed for primary or secondary tracheal tumours, 3 (13.6%) operations were performed for post-traumatic tracheal injuries and 2 (9.1%) resections for ILTS.
Table 1
| Variable | Number of patients (n=22) |
|---|---|
| Gender | |
| Male | 14 (63.6%) |
| Female | 8 (36.4%) |
| Age (years) | 54±21 (mean) |
| Site of disease | |
| Laryngotracheal | 13 (59.1%) |
| Tracheal | 9 (40.9%) |
| Aetiology | |
| ILTS | 2 (9.1%) |
| Post-intubation stenosis | 9 (40.9%) |
| Cancer | 4 (18.2%) |
| TEF | 4 (18.2%) |
| Post-traumatic injury | 3 (13.6%) |
| Pre-operative treatment | |
| No | 10 (45.5%) |
| Mechanical dilation | 10 (45.5%) |
| Laser ablation | 8 (36.4%) |
| Stenting | 3 (13.6%) |
| Post-traumatic neurological disorders | |
| Yes | 12 (54.5%) |
| No | 10 (45.5%) |
| Draining gastrostomy | 2 (9.1%) |
| Feeding jejunostomy | 2 (9.1%) |
| Neoadjuvant CHT | 1 (4.5%) |
| Cotton-Myer grade (n=13, LT stenosis) | |
| Grade I | 0 (0%) |
| Grade II | 11 (84.6%) |
| Grade III | 1 (7.7%) |
| Grade IV | 1 (7.7%) |
| McCaffrey stage (n=13, LT stenosis) | |
| Stage I | 0 (0%) |
| Stage II | 3 (23.1%) |
| Stage III | 8 (61.5%) |
| Stage IV | 2 (15.4%) |
ILTS, idiopathic laryngotracheal stenosis; TEF, tracheoesophageal fistula; LT, laryngotracheal; CHT, chemotherapy.
Twelve (54.5%) patients were affected by neurological disorders of traumatic aetiology. Twelve (54.5%) patients have been pre-operatively treated by conservative medical therapies, in particular mechanical dilation, laser ablation and stenting. One patient (4.5%) underwent neoadjuvant chemotherapy for a squamous cell carcinoma of third middle of the trachea and the same patient underwent post-operative radio-chemotherapy. Among patients with laryngotracheal stenosis, 11 (84.6%) had a grade II Cotton-Myer stenosis, 1 (7.7%) had a grade I and 1 (7.7%) patient had a grade IV Cotton-Myer stenosis; 3 (23.1%) patients were stage II, 8 (61.5%) patients were stage III and 2 (15.4%) were stage IV McCaffrey classification. Two out of 4 patients with TEF had a draining gastrostomy to avoid gastroesophageal reflux and a feeding jejunostomy to correct nutritional deficits.
All 18 (81.8%) operations were done with a collar cervical incision only. The other surgical access included 1 (4.5%) right posterolateral thoracotomy, 1 (4.5%) partial median sternotomy and 2 (9.1%) collar cervical incision combined with a total sternotomy. The main intra-operative findings are shown in Table 2. Ten (45.5%) patients underwent Pearson modified subglottic LTRR according to Liberman-Mathisen (20), 3 (13.6%) patients were operated on tracheal resection with primary anastomosis, 3 (13.6%) patients underwent single-stage TRR with direct esophageal closure, 3 (13.6%) patients underwent direct suture of the tracheal defect according to Angelillo-Mackinlay (24), 2 (9.1%) patients were operated on Grillo LTRR (21) with Liberman-mathisen plasty and 1 (4.5%) patient underwent thyroidectomy combined to Pearson modified subglottic laryngotracheal reconstruction according to Liberman-Mathisen for laryngeal infiltration from thyroid cancer.
Table 2
| Variable | Number of patients (n=22) |
|---|---|
| Surgical approach | |
| Collar cervical incision | 18 (81.8%) |
| Collar cervical incision + total sternotomy | 2 (9.1%) |
| Right postero-lateral thoracotomy | 1 (4.5%) |
| Partial median sternotomy | 1 (4.5%) |
| Tracheal release maneuver | |
| Digitally mobilization of the pre-tracheal plane | 21 (95.5%) |
| Video-mediastinoscopy mobilization of the pre-tracheal plane | 1 (4.5%) |
| Suprathyroid laryngeal release (Dedo-Fishman) | 17 (77.3%) |
| Type of surgery | |
| Pearson modified subglottic LTRR according to Liberman-Mathisen | 10 (45.5%) |
| Tracheal resection with primary anastomosis | 3 (13.6%) |
| Single-stage TRR with direct esophageal closure | 3 (13.6%) |
| Direct suture tracheal defect according to Angelillo-Mackinlay | 3 (13.6%) |
| Grillo LTRR with Liberman-Mathisen plasty | 2 (9.1%) |
| Thyroidectomy + Pearson modified subglottic LTRR according to Liberman-Mathisen | 1 (4.5%) |
| Length of tracheal resection (mm) | 32.17±6.15 (mean) |
| Extended tracheal resection | 4 (18.2%) |
| Anastomosis protection | 2 (9.1%) |
| Guardian stitch | 10 (45.5%) |
LTRR, laryngotracheal resection and reconstruction; TRR, tracheal resection and reconstruction.
The mean length of the resected trachea was 32.17±6.15 mm. Two reverse thymic fat pads were performed to protect extended cervico-mediastinic trachea resection-anastomosis. According to the definition of a resectional cut-off margin of 4 cm (25), 4 (18.2%) patients underwent extended tracheal resections.
The main post-operative outcomes of the population are summarized in Table 3. The postoperative hospitalization was 19.95±11.69 days, with a median value of 14 days. Six (27.3%) patients developed postoperative complications: 2 (9.1%) patients presented anastomotic leakage (treated by Vacuum Assisted Closure, V.A.C.®, therapy), 2 (9.1%) patients had dysphagia, one of which with a consequent inhalation pneumonia, 1 (4.5%) patient a bleeding with consequent cervical haematoma (which required a surgical treatment) and 1 (4.5%) patient had a temporary laryngeal palsy (treated by steroids). One perioperative death (4.5%) occurred after a single-stage extended third superior-middle TRR with direct esophageal closure. Weaning from mechanical ventilation was impossible for bilateral pneumonia, culminating in a recurrence of the TEF by day 12, complicated by ARDS and septic shock ended with death on 60th postoperative day. The incidence of postoperative complications was not significantly correlated to any potential risk factor analysed (gender, age, aetiology, previous treatment, neurological disorders, length of tracheal resection and so on, as showed in Table 4).
Table 3
| Variable | Number of patients (n=22) |
|---|---|
| Post-operative complications | 6 (27.3%) |
| Anastomotic leak | 2 (9.1%) |
| Dysphagia | 2 (9.1%) |
| Bleeding | 1 (4.5%) |
| Laryngeal palsy | 1 (4.5%) |
| Inhalation pneumonia | 2 (9.1%) |
| Sepsis | 1 (4.5%) |
| ARDS | 1 (4.5%) |
| Early recurrence of TEF | 1 (4.5%) |
| Late re-tracheostomy (recurrent inhalation pneumonia) | 1 (4.5%) |
| Peri-operative death | 1 (4.5%) |
| Post-operative hospitalization (days) | 19.95±11.69 |
| Adjuvant RT-CHT | 1 (4.5%) |
| Follow-up time (months) | 19.68±10.17 (mean) |
| Follow-up status | |
| Alive without disease | 19 (86.4%) |
| Alive with re-tracheostomy | 1 (4.5%) |
| Dead of disease (early recurrent TEF) | 1 (4.5%) |
| Dead of other causes | 1 (4.5%) |
| Tumor recurrence | 0 (0%) |
| Stenosis recurrence | 0 (0%) |
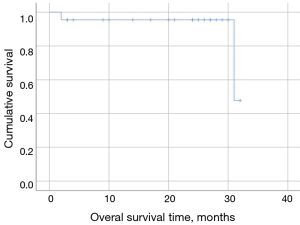
| Overall survival (months) | 30.16 (95% CI: 27.51–32.81) |
| 1-year OS | 95% |
| 2-year OS | 80% |
ARDS, acute respiratory distress syndrome; TEF, tracheoesophageal fistula; RT, radiotherapy; CHT, chemotherapy; OS, overall survival.
Table 4
| Variable | P value |
|---|---|
| Age | 0.622 |
| Gender | 0.267 |
| Aetiology | 0.757 |
| Pre-operative treatment | 0.508 |
| Neurological disorders | 0.888 |
| Site of disease (LT or TR) | 0.373 |
| Cotton-Myer grade | 0.873 |
| McCaffrey stage | 0.840 |
| Type of surgery | 0.428 |
| Length of LT/TR resection | 0.584 |
LT, laryngotracheal; TR, tracheal.
The mean follow-up time was 19.68±10.17 months. During the surveillance there was no evidence of either tumor recurrence or recurrent stenosis. At last follow-up, 20 (90.9%) patients were alive. Nineteen (86.4%) patients presented no evidence of disease while 1 (4.5%) patient was alive with a re-tracheostomy performed at 18 months after surgery for recurrent inhalation pneumonia. In addition to the single perioperative death, only another patient died (4.5%) for a cause not related to the underlying disease (death due to heart failure at 20 months after surgery for post-intubation laryngotracheal stenosis).
The mean OS was 30.16 months (95% CI: 27.51–32.81 months) (Figure 1). One- and 2-year OS was 95% and 80%, respectively. No statistically significant prognostic factors affecting OS were found at Cox regression model.
Discussion
Tracheal surgery is a challenge for thoracic surgeons even today, despite great progresses have been made in anaesthetic management and surgical techniques since the 1960s (2). The “fear” of tracheal surgery is due not only to the technical difficulties in itself, but also and above all to the high rate of morbidity and mortality correlated with the diseases of this airway tract. Nowadays, tracheal surgery is generally performed at high-volume, reference centres, by teams of specialized thoracic surgeons, anaesthesiologists, pulmonologists and otolaryngologists (11).
To date, in the absence of distant metastasis and if local resectability is provided, surgical resection followed by primary reconstruction and adjuvant radiotherapy in case of microscopic residual disease is the best curative treatment for tracheal cancer (3). Patients treated with surgical resection have better prognosis than unresected ones, with a 5- and 10-year survival rates of 50% and 35%, respectively, compared to 5–15% and 6–7%, respectively, of patients not undergoing surgery (26,27). It has finally passed from a “nihilistic attitude based on ignorance”, as reproached by Licht and colleagues (26), to a positive overture to surgical management of tracheal cancers thanks to a progressive acquired knowledge of operative techniques. Gaissert and coworkers (4) found that locoregional tumor is the most important determinant of resectability. Complete resection, negative airway margins, and adenoid cystic histology affect long-term survival in a statistically significant way. Conversely, survival is independent of the type of resection. Our data corroborate this finding, in fact the type of surgery did not influence post-operative complications and survival in a statistically significant manner.
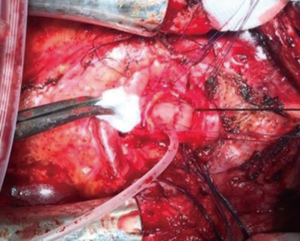
Controversies are still present among surgeons about the length of tracheal resectability. Extended tracheal resections are generally considered to be burdened by a greater rate of post-operative complications than standard resections, whose cut-off margin can be accounted a length of less than 4 cm. In our cohort, post-operative complications and OS were not related to the length of tracheal resection, confirming the importance of careful release maneuvers to minimize devascularization and to achieve tension-limited anastomosis (25,28). Additionally, the coverage of tracheal anastomosis suture line with a vascularized tissue flap to prevent erosion of contiguous brachiocephalic vessels is still a matter of debate (29). We have protected extended cervico-mediastinic tracheal resection with a reverse thymic fat pad in two cases, one of which underwent neoadjuvant chemotherapy and post-operative chemo-radiotherapy for a squamous cell carcinoma (Figure 2A,B). The patient had good short- and long-term outcomes, suggesting that extended tracheal resection following induction chemotherapy could be safely performed. Reverse thymic fat pad proves to be a good tool for protecting the suture line. It has been demonstrated that omentum enhances blood supply, processing an angiogenic factor, and it improves healing by new fibroblast growth (30). According to the “omentum model”, we suppose that thymic fat flap might confer similar biological advantages and thus, add an advantage in terms of better throphism of tracheal anastomosis suture line.
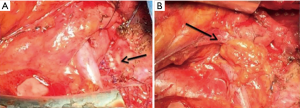
Conservative medical treatments such as mechanical dilation, laser ablation and stenting have a limited and ephemeral role in the treatment of tracheal stenosis due to high recurrence rates (31,32). In sight of this, patients should be considered for definitive surgical operation. Subglottic stenosis provides the greatest challenge for single-stage LTRR (33,34). Subglottic larynx represents the narrowest segment of airway and it is close to the vocal cords and recurrent laryngeal nerve insertion. In addition, these lesions are frequently associated with side-to-side or lateral narrowing of the subglottic space, decreasing the luminal diameter tight after anastomosis, with a symptomatic relief often unsatisfactory (20). In this case, a tailored cricoplasty added to the standard technique of anterior cricoid resection and eventually the cricoid plate mucosectomy (Grillo’s procedure) improves short- and long-term outcomes of reconstructive subglottic stenosis (33) (Figure 3).

We obtained excellent long-term results, in fact none of the patients affected by subglottic stenosis developed a late stricture recurrence. Only one patient affected by neurological disorders operated on LTRR for a first and second tracheal ring post-intubation recurrent stenosis has needed re-tracheostomy for repeated inhalation pneumonia at 18 months after surgery. Our results are in line with those reported by other authors, who described good to excellent long term outcome in more than 90% of patients with perioperative mortality under 1–2% (21,34-36). Grillo reported good to excellent results from six months to five and one-half years later LTRR in sixteen out of eighteen patients operated on for subglottic laryngeal and upper tracheal stenosis (21). The reconstruction in a patient with burned airway failed and one patient treated for ILTS had needed a T tube placement for recurrent stenosis. Marulli and colleagues described excellent to satisfactory results in more than 90% of patients who underwent single-stage LTRR to treat benign laryngotracheal strictures (35). In particular, long-term results were excellent to satisfactory in 36 patients (97.3%) and unsatisfactory in one (2.7%), who presented late 40% restenosis with severe hoarseness and episodic dyspnoea, requiring frequent laser ablations and dilatations. Liberman and Mathisen, in their initial report of tailored cricoplasty, stated that none of the 18 patients undergoing surgery required reoperation, tracheostomy, or readmission to the hospital during the entire follow-up period (20). All patients affirmed that they were satisfied and would choose surgery again. In one of the largest series ever published of patients undergoing LTRR for benign stenosis, D’Andrilli and co-authors showed definitive excellent or good results in 94.5% of patients (36).
According to Sanchez-Lorente and colleagues (23) we performed airway reconstruction under apnoeic hyperoxygenation to provide an adequate oxygenation during the apnoeic time period of anastomosis by an increase of intrapulmonary oxygen storage (37) (Figure 4).
Analysing the whole study cohort, we observed no significant correlation between the incidence of postoperative complications and any potential risk factor investigated. However, the very small sample size represented a no negligible bias. Patients with the added risk deriving from post-coma neuropsychiatric disorders showed no increased rates of complication and surgical failure. We reported post-operative complications in 27.3% of patients, a rate even better compared to incidence reported in literature of about 35% (35). All complications were successfully treated, with no late sequelae, except one patient who developed early TEF recurrence, complicated by ARDS and septic shock culminating in death.
TEFs are life-threatening conditions which require an operative reconstructive management to eliminate the fistula and restore a functional airway, because spontaneous healing is very rare (38). Several surgical options have been proposed, including direct suture closure of both the tracheal and esophageal defects with or without pedicled muscle flaps (39,40), tracheal closure using an esophageal patch (41), segmental tracheal resection and primary anastomosis with direct esophageal closure (42,43), a combined surgical and endoscopic approach (44), and a two-stage approach with esophageal diversion and primary closure of the tracheal defect (42,45). Nonetheless, the optimal timing and strategy of TEFs repair remain controversial. In our practice we generally performed TRR or LTRR with primary esophageal closure, according to the previous results of Macchiarini and colleagues, which reported less common complications (7% vs. 38%) and better long-term results (93% vs. 65%) as compared with other procedures (43). Moreover, all but one of our patients had post-intubation injury, the most common indication for tracheal resection. In case of absence of circumferential damage and airway stenosis, direct repair of the tracheal and esophageal defects, with interposition of robust vascularized tissue, is simpler and effective (13). We applied the anterior approach as described by Grillo, to avoid extensive devascularization and allow a complete exposure of the entire tracheoesophageal damage (22). In extensive TEFs, a draining gastrostomy might be useful to avoid gastroesophageal reflux, as well as a feeding jejunostomy to ensure an adequate nutritional support (43). Some reports without long-term follow-up support initial management of non-malignant TEF with endoluminal stents, usually in the esophageal localization (46). Nevertheless, clinical experience has shown a large number of failures of this technique over the years, particularly a real risk for exacerbation is demonstrated, as stents have been involved in the creation of giant TEFs (47). Perioperative mortality in our series was 4.5%, which is similar to the rates reported in literature, between 3.2% to 29.6% (12,39,42-45). Therefore, even if technically challenging, we advocate a prompt surgical repair of acquired benign TEFs.
Tracheal injuries are life-threating conditions, requiring an early diagnosis and a prompt treatment to reduce morbidity and mortality (48). Posterior tracheal wall lacerations are rare and generally iatrogenic, occurring during intubation (49). When the tear involves the third distal of the trachea, the best surgical exposition is obtained through a thoracotomy, whereas if the laceration is located in the superior-middle third of the trachea, a cervicotomy should be performed. In our experience we managed only three membranous tracheal wall lacerations, two post-intubation and one caused by a road trauma. According to Angelillo-Mackinlay (24), we fashioned a Kocher cervicotomy and a longitudinal tracheotomy on the anterior wall to reach and suture the membranous wall from the inside. All the surgical procedures proved effective in restoring airway integrity and with no long-term sequelae. Several previous larger series had showed similar data, confirming the safety, reproducibility and reliability of the transcervical-transtracheal endoluminal repair of membranous injuries, avoiding the possible complications of tracheal posterior dissection (48,50,51).
We observed a 1- and 2-year OS of 95% and 80%, respectively. None of the neoplastic patients developed local or distant recurrence. However, regarding the prognostic factors affecting long-term outcome, none of the pre-, intra- and post-operative characteristics have resulted as predictors of survival.
This study presents some major weaknesses as well. First of all, it is a single centre, retrospective, non-randomised series affected by selection bias. Secondly, the small sample size, due to the low rate of tracheal diseases susceptible to surgical treatment. Moreover, an analysis for subgroups was not possible due to the great heterogeneity of the diseases analysed, with a very small sample size of each subgroup, and this may reduce the effectiveness of our results. Finally, a longer follow-up would be needed to demonstrate the safety and the efficacy of tracheal surgery in terms of long-term outcomes. Nevertheless, as far as we know, this is one of the largest series reporting a study period of only 30 months.
In conclusion, taking into account the peculiar anatomical features of the larynx and the trachea, the relative rarity of tracheal diseases and the technical difficulties of surgery of this airway tract, which is burdened by potentially severe complications, a careful and extensive preoperative assessment of the patient is mandatory. It is important to emphasize that tracheal surgery should be performed by experienced surgeons in specialized, high-volume centres, where a knowledgeable multidisciplinary team consisting of thoracic surgeons, anaesthesiologists, otolaryngologists, pulmonologists and intensivists work together to manage these patients’ care. Our preliminary experience confirm that in skilful hands tracheal surgery appears feasible, safe and effective, even in the most challenging subset of laryngotracheal resections, allowing excellent long-term outcomes.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Francesco Zaraca, Reinhold Perkmann, Luca Bertolaccini and Roberto Crisci) for the series “Thoracic Surgery Without Borders” published in Current Challenges in Thoracic Surgery. The article has undergone external peer review.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ccts.amegroups.com/article/view/10.21037/ccts.2020.01.04/coif). The series “Thoracic Surgery Without Borders” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was evaluated by the Institutional Review Board (IRB) of San Camillo Forlanini Hospital and, as this was a retrospective review for service evaluation (within an audit approved by our Surgical Department) and there was no modification in patients’ care (no prospective randomized study), we did not need the final ethical approval of our IRB. Written informed consent was obtained from the patients for publication of this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Belsey R. Resection and reconstruction of the intrathoracic trachea. Br J Surg 1950;38:200-5. [Crossref] [PubMed]
- Grillo HC. Development of tracheal surgery: a historical review. Part 1: techniques of tracheal surgery. Ann Thorac Surg 2003;75:610-9. [Crossref] [PubMed]
- Honings J, Gaissert HA, van der Heijden HF, et al. Clinical aspects and treatment of primary tracheal malignancies. Acta Otolaryngol 2010;130:763-72. [Crossref] [PubMed]
- Gaissert HA, Grillo HC, Shadmehr MB, et al. Long-term survival after resection of primary adenoid cystic and squamous cell carcinoma of the trachea and carina. Ann Thorac Surg 2004;78:1889-96. [Crossref] [PubMed]
- Regnard JF, Fourquier P, Levasseur P. Results and prognostic factors in resections of primary tracheal tumors: a multicenter retrospective study. The French Society of Cardiovascular Surgery. J Thorac Cardiovasc Surg 1996;111:808-13. [Crossref] [PubMed]
- Gaissert HA, Honings J, Grillo HC, et al. Segmental laryngotracheal and tracheal resection for invasive thyroid carcinoma. Ann Thorac Surg 2007;83:1952-9. [Crossref] [PubMed]
- Cooper JD, Grillo HC. Analysis of problems related to cuffs on intratracheal tubes. Chest 1972;62:21S-27S. [Crossref] [PubMed]
- Grillo HC, Donahue DM. Postintubation tracheal stenosis. Chest Surg Clin N Am 1996;6:725-31. [PubMed]
- Grillo HC, Mark EJ, Mathisen DJ, et al. Idiopathic laryngotracheal stenosis and its management. Ann Thorac Surg 1993;56:80-7. [Crossref] [PubMed]
- Terada M, Hotoda K, Toma M, et al. Surgical management of congenital tracheal stenosis. Gen Thorac Cardiovasc Surg 2009;57:175-83. [Crossref] [PubMed]
- Sihag S, Wright CD. Prevention and management of complications following tracheal resection. Thorac Surg Clin 2015;25:499-508. [Crossref] [PubMed]
- Shen KR, Allen MS, Cassivi SD, et al. Surgical Management of Acquired Nonmalignant Tracheoesophageal and Bronchoesophageal Fistulae. Ann Thorac Surg 2010;90:914-8. [Crossref] [PubMed]
- Muniappan A, Wain JC, Wright CD, et al. Surgical treatment of nonmalignant tracheoesophageal fistula: a thirty-five year experience. Ann Thorac Surg 2013;95:1141-6. [Crossref] [PubMed]
- Juvekar NM, Deshpande SS, Nadkarni A, et al. Perioperative management of tracheobronchial injury following blunt trauma. Ann Card Anaesth 2013;16:140-3. [Crossref] [PubMed]
- Kiser AC, O'Brien SM, Detterbeck FC. Blunt tracheobronchial injuries: treatment and outcomes. Ann Thorac Surg 2001;71:2059-65. [Crossref] [PubMed]
- McCaffrey TV. Classification of laryngotracheal stenosis. Laryngoscope 1992;102:1335-40. [Crossref] [PubMed]
- Myer CM 3rd, O'Connor DM, Cotton RT. Proposed grading system for subglottic stenosis based on endotracheal tube sizes. Ann Otol Rhinol Laryngol 1994;103:319-23. [Crossref] [PubMed]
- Dedo HH, Fishman NH. Laryngeal release and sleeve resection for tracheal stenosis. Ann Otol Rhinol Laryngol 1969;78:285-96. [Crossref] [PubMed]
- Pearson FG, Cooper JD, Nelems JM, et al. Primary tracheal anastomosis after resection of the cricoid cartilage with preservation of recurrent laryngeal nerves. J Thorac Cardiovasc Surg 1975;70:806-16. [Crossref] [PubMed]
- Liberman M, Mathisen DJ. Tailored cricoplasty: an improved modification for reconstruction in subglottic tracheal stenosis. J Thorac Cardiovasc Surg 2009;137:573-8. [Crossref] [PubMed]
- Grillo HC. Primary reconstruction of airway after resection of subglottic laryngeal and upper tracheal stenosis. Ann Thorac Surg 1982;33:3-18. [Crossref] [PubMed]
- Grillo HC, Moncure AC, McEnany MT. Repair of inflammatory tracheoesophageal fistula. Ann Thorac Surg 1976;22:112-9. [Crossref] [PubMed]
- Sanchez-Lorente D, Iglesias M, Rodríguez A, et al. The pumpless extracorporeal lung membrane provides complete respiratory support during complex airway reconstructions without inducing cellular trauma or a coagulatory and inflammatory response. J Thorac Cardiovasc Surg 2012;144:425-30. [Crossref] [PubMed]
- Angelillo-Mackinlay T. Transcervical repair of distal membranous tracheal laceration. Ann Thorac Surg 1995;59:531-2. [Crossref] [PubMed]
- Hecker E, Volmerig J. Extended tracheal resections. Thorac Surg Clin 2014;24:85-95. [Crossref] [PubMed]
- Licht PB, Friis S, Pettersson G. Tracheal cancer in Denmark: a nationwide study. Eur J Cardiothorac Surg 2001;19:339-45. [Crossref] [PubMed]
- Honings J, van Dijck JA, Verhagen AF, et al. Incidence and treatment of tracheal cancer: a nationwide study in the Netherlands. Ann Surg Oncol 2007;14:968-76. [Crossref] [PubMed]
- Macchiarini P, Altmayer M, Go T, et al. Interdisciplinary Intrathoracic Tumor Task Force. Technical innovations of carinal resection for nonsmall-cell lung cancer. Ann Thorac Surg 2006;82:1989-97. [Crossref] [PubMed]
- Grillo HC. Surgical treatment of postintubation tracheal injuries. J Thorac Cardiovasc Surg 1979;78:860-75. [Crossref] [PubMed]
- Muehrcke DD, Grillo HC, Mathisen DJ. Reconstructive airway operation after irradiation. Ann Thorac Surg 1995;59:14-8. [Crossref] [PubMed]
- Brichet A, Verkindre C, Dupont J, et al. Multidisciplinary approach to management of postintubation tracheal stenoses. Eur Respir J 1999;13:888-93. [Crossref] [PubMed]
- Valdez TA, Shapshay SM. Idiopathic subglottic stenosis revisited. Ann Otol Rhinol Laryngol 2002;111:690-5. [Crossref] [PubMed]
- Liberman M, Mathisen DJ. Treatment of idiopathic laryngotracheal stenosis. Semin Thorac Cardiovasc Surg 2009;21:278-83. [Crossref] [PubMed]
- Macchiarini P, Verhoye JP, Chapelier A, et al. Partial cricoidectomy with primary thyrotracheal anastomosis for postintubation stenosis. J Thorac Cardiovasc Surg 2001;121:68-76. [Crossref] [PubMed]
- Marulli G, Rizzardi G, Bortolotti L. Single-staged laryngotracheal resection and reconstruction for benign strictures in adults. Interact Cardiovasc Thorac Surg 2008;7:227-30. [Crossref] [PubMed]
- D’Andrilli A, Maurizi G, Andreetti C, et al. Long-term results of laryngotracheal resection for benign stenosis from a series of 109 consecutive patients. Eur J Cardiothorac Surg 2016;50:105-9. [Crossref] [PubMed]
- Frumin MJ, Epstein RM, Cohen G. Apneic oxygenation in man. Anesthesiology. 1959;20:789-98. [Crossref] [PubMed]
- Dartevelle P, Macchiarini P. Management of acquired tracheoesophageal fistula. Chest Surg Clin N Am 1996;6:819-36. [PubMed]
- Mathisen DJ, Grillo HC, Wain JC, et al. Management of acquired nonmalignant tracheoesophageal fistula. Ann Thorac Surg 1991;52:759-65. [Crossref] [PubMed]
- Mangi AA, Gaissert HA, Wright CD, et al. Benign bronchoesophageal fistula in the adult. Ann Thorac Surg 2002;73:911-5. [Crossref] [PubMed]
- Bartlett RH. A procedure for the management of acquired tracheoesophageal fistula in ventilatory patients. J Thorac Cardiovasc Surg 1976;71:89-95. [Crossref] [PubMed]
- Marzelle J, Dartevelle P, Khalife J, et al. Surgical management of acquired postintubation trachea-oesophageal fistulas: 27 patients. Eur J Cardiothorac Surg 1989;3:499-502. [Crossref] [PubMed]
- Macchiarini P, Verhoye JP, Chapelier A, et al. Evaluation and outcome of different surgical techniques for postintubation tracheoesophageal fistulas. J Thorac Cardiovasc Surg 2000;119:268-76. [Crossref] [PubMed]
- Freire JP, Feijó SM, Miranda L, et al. Tracheo-esophageal fistula: combined surgical and endoscopic approach. Dis Esophagus 2006;19:36-9. [Crossref] [PubMed]
- Cherveniakov A, Tzekov C, Grigorov GE, et al. Acquired benign esophago-airway fistulas. Eur J Cardiothorac Surg 1996;10:713-6. [Crossref] [PubMed]
- Eleftheriadis E, Kotzampassi K. Temporary stenting of acquired benign tracheoesophageal fistulas in critically ill ventilated patients. Surg Endosc 2005;19:811-5. [Crossref] [PubMed]
- Han Y, Liu K, Li X, et al. Repair of massive stent-induced tracheoesophageal fistula. J Thorac Cardiovasc Surg 2009;137:813-7. [Crossref] [PubMed]
- Mussi A, Ambrogi MC, Ribechini A, et al. Acute major airway injuries: clinical features and management. Eur J Cardiothorac Surg 2001;20:46-51. [Crossref] [PubMed]
- Kumar SM, Pandit SK, Cohen PJ. Tracheal laceration associated with endotracheal anaesthesia. Anesthesiology 1977;47:298-9. [Crossref] [PubMed]
- Mussi A, Ambrogi MC, Menconi G, et al. Surgical approaches to membranous tracheal wall lacerations. J Thorac Cardiovasc Surg 2000;120:115-8. [Crossref] [PubMed]
- Lancelin C, Chapelier AR, Fadel E, et al. Transcervical-Transtracheal endoluminal repair of membranous tracheal disruptions. Ann Thorac Surg 2000;70:984-6. [Crossref] [PubMed]
Cite this article as: Jaus MO, Mastromarino MG, Cardillo G. Tracheal surgery: still a challenge or a reality for thoracic surgeons?—A 30-month single-centre experience. Curr Chall Thorac Surg 2020;2:14.