
Closure of late bronchopleural fistula with atrial septal occluder
Introduction
Bronchopleural fistula (BPF) is a severe and often dramatic complication after lung resection. Although the incidence is dropped over the last years reaching near to 4%, it remains a challenging situation for thoracic surgeons (1). The treatment can include several strategies, ranging from “wait and see” policy, medical therapy, redo surgery and endobronchial devices deployment. Recently, devices designed for closure of cardiac defects have been successfully used to repair BPF (2). We report a case of a patient with a late BPF successfully treated with atrial septal occluder after several failed attempts with conventional techniques.
Case presentation
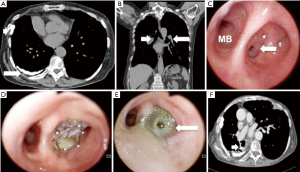
A 74-year-old woman with a history of breast and bowel cancer underwent right lower lobectomy for a lung cancer. Previous cancers were treated with surgery and adjuvant radiotherapy (breast) and chemotherapy (bowel); after radiotherapy she developed a large right fibrothorax (Figure 1A white arrows) and consequently to chemotherapy her body mass index (BMI) decreased. At preoperative computed tomography (CT) scan diffuse bronchial calcifications (Figure 1B white arrows) were also detected and at surgery the closure of bronchial stump resulted very hard and it was distally performed; however at the end of operation no air leakage from bronchial stump was observed. The postoperative course was characterized by prolonged parenchymal air leaks and not complete re-expansion of lung; however, bronchoscopy didn’t show any signs of BPF and patient was discharged after 15 days with Heimlich valve and the drainage was removed in 26th postoperative day. After one year the patient presented low degrees of fever, metallic taste and muco-purulent expectoration. A CT scan showed a purulent encapsulated pleural effusion and bronchoscopy showed a minimal fistula in a subsegment of right lower bronchial stump. Under CT vision a pleural drainage was inserted to clean the cavity and, through fiber bronchoscopy, fibrin glue was placed to occlude the BPF, with success. The patient remained asymptomatic for another one year and half when she presented again signs of infection. At bronchoscopy the BPF was larger (Figure 1C) and the cavity in the pleural space full of purulent fluid. She newly underwent drainage of cavity and bronchoscopic insertion of fibrin glue; however the dimension and the rigidity of fistula due to calcifications didn’t allow its closure. We tried to insert a one-way valve in the fistula (Figure 1D), but the patient expectorated it after 12 hours. According to recent publications about BPF closure with atrial septal occluder we decided to try this strategy. Under deep sedation, the patient underwent rigid bronchoscopy and, under direct and fluoroscopic view, the device (Figulla Flex II, Occlutech) was correctly delivered and placed. At bronchoscopy and CT scan control (Figure 1E,F, white arrows) performed the day after, BPF was completely closed and the patient was successfully discharged. She remained asymptomatic for two years when she died because of recurrence of breast cancer.
Discussion
BPF is a rare but often severe complication after major pulmonary resections. Several risk factors have been reported to increase the risk of BPF as administration of induction and adjuvant chemo-radiotherapy, diabetes, impaired BMI, infections, postoperative mechanical ventilation (3). Surgery remains the gold standard treatment in case of massive early BPF and to treat the infective complications in late presentation. Also several endoscopic techniques as administration of fibrin glues, sealants, silver nitrate and albumin, staminal cells and placement of covered stents and endobronchial valves have been proposed with different degree of success (4). Recently, it has been implemented the use of devices designed to treat atrial septal defects to occlude BPF (5) but, differently from our case, in particular after pneumonectomy and in early postoperative period.
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/ccts.2019.08.05). FV serves as an unpaid editorial board member of Current Challenges in Thoracic Surgery from Mar 2019 to Mar 2021. The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Jichen QV, Guangyu C, Gening J, et al. Risk factor comparison and clinical analysis of early and late bronchopleural fistula after non-small cell lung cancer surgery. Ann Thorac Surg 2009;88:1589-93. [Crossref] [PubMed]
- Tedde ML, Scordamaglio PR. Endobronchial closure of total bronchopleural fistula with Occlutech Figulla ASD N Device. Ann Thorac Surg 2009;88:e25-6. [Crossref] [PubMed]
- Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg 2001;13:3-7. [Crossref] [PubMed]
- Lois M, Noppen M. Bronchopleural fistula: an overview of the problem with special focus on endoscopic management. Chest 2005;128:3955-65. [Crossref] [PubMed]
- Fruchter O, Kramer MR, Dagan T, et al. Endobronchial closure of bronchopleural fistulae with Amplatzer devices: our experience and literature review. Chest 2011;139:682-7. [Crossref] [PubMed]
Cite this article as: Poggi C, Diso D, Tanzilli G, Venuta F, Anile M. Closure of late bronchopleural fistula with atrial septal occluder. Curr Chall Thorac Surg 2019;1:12.


