Clinical consensus on preoperative pulmonary function assessment in patients undergoing pulmonary resection (first edition)
Introduction
While surgery is an important treatment option for lung diseases, its value can be compromised in patients with impaired lung function. The decreased lung ventilation or diffusion function in these patients increases the risk of perioperative complications and affects long-term survival. Therefore, the extent of resection should be defined according to disease stage; based on the pulmonary function, the risk of perioperative complications and long-term quality of life should be evaluated and predicted before surgery. Thus, the feasibility of surgical treatment and the extent of resection should be decided after weighing the pros and cons.
Since Borelli first measured expiratory volume in 1679, the development of lung function tests has evolved tremendously. Pulmonary function testing has been applied in thoracic surgery for more than 50 years. In 1971, Boushy and colleagues (1) found one second forced expiratory volume (FEV1) can be used as an essential indicator of surgical tolerance before a thoracic surgery. Later, in a study enrolling 2,340 patients by Miller (2), when the results of a comprehensive analysis of pulmonary functions including FEV1, maximum voluntary ventilation (MVV), and forced expiratory flow rate from 25% to 75% (FEF25–75) were applied, a more precise method of selecting patients for various types of pulmonary resection has resulted in a lower mortality. In 1987, Bechard and Wbtstein (3) concluded that exercise was an essential criterion in the preoperative evaluation of patients for pulmonary surgery as cardiopulmonary exercise testing (CPET) could effectively simulate the status of patients after pneumonectomy by testing cardiac and pulmonary load and function simultaneously; meanwhile, a maximal exercise O2 consumption (VO2max) less than 10 mL/kg/min was significantly associated with morbidity and mortality. In 1988, Ferguson et al. (4) demonstrated the role of diffusing capacity of the lung for carbon monoxide (DLCO) in predicting the prognosis of postoperative patients with lung cancer. Since then, FEV1 and DLCO have been used as standard preoperative pulmonary evaluation of the lung resection candidates. In 1999, Wyser et al. (5) developed an algorithm that incorporated FEV1, DLCO, and VO2max as well as their respective ppo values for the preoperative functional evaluation, which further decreased the complication rate by 50%. Since then, the British Thoracic Society (6), the European Respiratory Society (ERS) and the European Society of Thoracic Surgeons (ESTS) (7), and the American College of Chest Physicians (ACCP) (8) have issued relevant guidelines to guide preoperative pulmonary function testing.
The purpose of preoperative pulmonary function assessment is to accurately assess lung function by using non-invasive or minimally invasive methods, to predict the risk of perioperative complications and the long-term survival of patients after radical surgery and to make an informed decision on the extent of surgical resection. Based on the assessment results, effective prevention and control measures can be taken to reduce complications and improve the long-term quality of life.
This consensus document aligns existing literature and international clinical guidelines and attempts to offer clinicians with guidance on the standardized preoperative assessment of lung function.
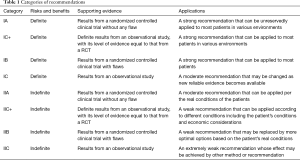
The categories of recommendations are shown in Table 1.
Full table
Ventilation and diffusing capacity of the lungs
Spirometry
The FEV1 and the predicted postoperative (PPO)-FEV1 are the main parameters for measuring the ventilation function. The decline in FEV1 and PPO-FEV1 suggests an increased risk of postoperative complications and perioperative mortality. According to the standard guidelines (6,9), the screening of surgical candidates is mainly based on the absolute value of FEV1: patients are suitable for pneumonectomy if FEV1 is greater than 2 L and for lobectomy is FEV1 is greater than 1.5 L. No further physiologic evaluation is required for these patients.
FEV1 is highly diverse among individual patients, and it is not feasible to screen surgical patients based on the absolute value of FEV1 alone. A model was established based on large data sets, including patients’ gender, height, weight, age, and other factors to estimate the expected lung function. The introduction of FEV1% provided an excellent solution to account for individual differences. Most studies have shown that a PPO-FEV1 of less than 40% predicted suggests an increase in perioperative complication rates, which are 16–50% in the literature (10-14). Nakahara et al. (15) found a mortality rate as high as 60% when ppo-FEV1 was <30% predicted. Therefore, a PPO-FEV1 of less than 40% of predicted is typically used as a cut-off value to categorize operations as “high risk”.
The cut-off screening value for ventilation function changes with the advances in relevant research. Research has suggested that FEV1 is an independent risk factor for surgery: The incidence of perioperative respiratory complications was 43% in patients with preoperative FEV1 <35% predicted and was only 12% in patients with FEV1 >60% predicted. In a study of 1,046 patients by Ferguson et al. (16), FEV1 was an independent risk factor for predicting perioperative mortality due to pulmonary complications (OR, 1.1; FEV1 decreased by 10%) and also a risk factor for perioperative deaths from cardiovascular complications (OR, 1.13; FEV1 decreased by 10%). In a cohort of 1,239 patients, Licker et al. (17) found FEV1 could be used to predict surgical complications, with FEV1 <60% predicted being the optimal predictor of perioperative mortality and respiratory morbidity.
Diffusing capacity test
The diffusion capacity is mainly assessed by measuring the DLCO. In 1988, Ferguson et al. (4) found that DLCO was an independent predictor of complications and death after lung resection: patients with a DLCO of less than 60% predicted had a postoperative respiratory complication rate of 40% and a perioperative mortality rate of 25%. Berry et al. (18) studied 340 patients and found the perioperative mortality rate was 5% and the complication rate was 48% in patients having either FEV1 or DLCO below 60% predicted after lobectomy, and both FEV1 and DLCO were independent risk factors. Cerfolio et al. (19) enrolled 906 patients and also confirmed that both DLCO and FEV1 were important predictors; meanwhile, they proposed that DLCO/VA% was another important predictor of postoperative complications after pneumonectomy. Further studies have demonstrated the association between DLCO and long-term survival of patients after lung resection. In a study conducted by Liptay et al. (20), patients were divided into different DLCO groups after adjusted for FEV1, and the results showed that the long-term survival rates dropped with the decrease of DLCO. Ferguson et al. (21) found on univariate analysis that the hazard ratio between DLCO of <60% group and DLCO of ≥80% group was 1.35, and multivariate analysis revealed DLCO was an independent predictor of long-term survival.
For patients undergoing pulmonary surgery, the current international guidelines recommend the use of both FEV1 and DLCO for evaluation, along with the calculation of PPO-FEV1 and PPO-DLCO. According to the current ERS/ESTS guidelines, FEV1 and DLCO are still recommended during the first stage of qualifying patients for lung resection. If both of them are greater than 80% predicted, no further evaluation is needed, and elective surgery can be planned. According to the ACCP guidelines, however, the calculation and evaluation of PPO-FEV1 and PPO-DLCO are required based on the resection extent; if both of them are greater than 60% predicted, no further evaluation is required, and surgery (e.g., total pneumonectomy) can be performed. The calculation of PPO-FEV1 and PPO-DLCO uses the number of functioning lung segments and the number of lung segments to be removed. Before a lobectomy, segmentectomy, combined segmentectomy/subsegmentectomy, and/or combined lobectomy, the lung function can be assessed by calculating PPO-FEV1 and PPO-DLCO according to the calculation methods provided in the guidelines. The actual situations can be a lot more complicated. For instance, the lung tissues to be removed by surgical or non-surgical means may have inconsistent functions (the presence of non-functioning area or heterogeneous lesions, especially in patients presented with heterogeneously distributed diffuse lung disease). Under such conditions, CT or radionuclide perfusion imaging should be performed to assess the number of functioning lung units, to predict PPO-FEV1 and PPO-DLCO more accurately. It has been found that preoperative CT three-dimensional reconstruction can be used for the evaluation of PPO-FEV1 and PPO-DLCO (22). Le Roux et al. (23) used gallium-68 and strontium-99-macroaggregated polymeric albumin lung perfusion PET/CT to detect 22 patients undergoing lung surgery and performed preoperative and postoperative pulmonary function tests and follow-up; they found the lung function in different lobes significantly differed among different patients.
Recommendations
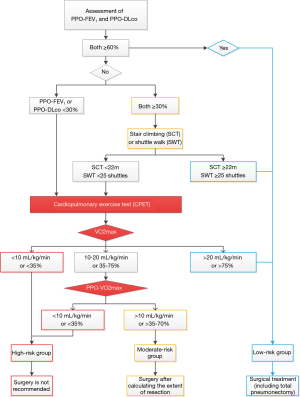
For all patients who may undergo radical surgery, it is recommended to measure both FEV1 and DLCO and calculate and evaluate PPO-FEV1 and PPO-DLCO according to the resection sizes (Category IB).
For all patients who may undergo radical surgery, no further evaluation is required if the PPO-FEV1 and PPO-DLCO are greater than 60% predicted according to the resection size, and surgical resection can be scheduled (Category IC).
For all patients who may undergo radical surgery, low-technology exercise tests [including stair climbing test (SCT) and shuttle walk test (SWT)] are recommended if either PPO-FEV1 or PPO-DLCO is less than 60% predicted and both are greater than 30% predicted according to the resection size (Category IC).
For all patients who may undergo radical surgery, CPET is recommended to measure VO2max if either PPO-FEV1 or PPO-DLCO is less than 30% predicted according to the resection size (Category IB).
Low-technology exercise tests
SCT
SCT is a simple pulmonary exercise testing that requires no special equipment or staffing. Patients have to mobilize a large number of muscles during SCT. Compared with other sports, SCT exerts a certain pressure on the patient and can, to some extent, reflect the cardiopulmonary function. In 1987, Bolton et al. (24) confirmed the association between SCT and lung function: FVE1 was >1.7 L in patients who could climb three flights of stairs and >2.0 L in those who could climb five flights of stairs. However, SCT has certain limitations: factors including climbing speed, the height of stairs, and body weight of patients can dramatically affect the results. Thus, efforts have been made to standardize SCT.
- Changing the floor count to the total height of the stairs climbed. In a series of 160 patients, Brunelli et al. (25) found that the postoperative cardiopulmonary complication rate was 6.5% in patients reached an altitude of 14m and 50% in those who could only climb less than 12 m. A larger study in 2008 showed the cardiopulmonary complication rate of patients whose climbing altitude was lower than 12 m was two times of that in patients who could climb more than 22 m, along with 13 times higher mortality rate (26). The climbing altitude was significantly correlated with the results of CPET: 56% of patients with a climbing altitude of less than 14 m had a VO2max of less than 15 mL/kg/min, while 98% of patients with an altitude of more than 22 m had a VO2max of greater than 15 mL/kg/min (27).
- Adjusting for body weight and climbing speed. In a recent study, Novoa et al. (28) established a modified formula after the patients’ body weight, and climbing speed was adjusted, which represented a meaningful exploration in low-technology exercise tests.
In summary, stair climbing can offer general information on the cardiopulmonary function; for patients with subnormal lung function, the cardiopulmonary function test is more feasible.
SWT
SWT is another simple cardiopulmonary function test, during which a patient is asked to walk back and forth between two markers 10 meters apart. Walking speed is increased each minute in a graded fashion and paced by an audio signal. The test is stopped when the patient is too breathless to maintain speed. Singh et al. (29) found an inability to complete 25 shuttles (250 m) on two occasions suggested a VO2max of <10 mL/kg/min. Win et al. (30) suggested that the walking distance had a certain correlation with VO2max, although SWT might underestimate VO2max. Benzo et al. (31) demonstrated a definite linear correlation between SWT results and the VO2max: the average VO2max was 55 mL/kg/min in 55 subjects who had completed 25 shuttles. Although SWT is not a common test in clinical practice and its value in assessing clinical lung function is slightly lower than that of SCT, it can still be used as a supplementary test to improve the accuracy of low-technology exercise tests.
6-minute walking test (6MWT)
There are currently no detailed definitions of the methods and standards of 6MWT. Few studies have investigated 6MWT, and the results were significantly inconsistent. Currently, 6MWT is not recommended for preoperative lung function assessment in most international guidelines. A recent study (32) described a modified 6MWT and proposed that measuring the heart rate and oxygen saturation during 6MWT could improve the accuracy of preoperative lung function assessment. Such studies shed new light on the improvements in low-technology exercise tests.
Recommendations
For all patients who may undergo radical surgery, CPET is recommended to measure VO2max if the walking distance is less than 25 shuttles (<400 m) during SWT or if the climbing altitude is below 22 m during SCT (Category IC).
For all patients who may undergo radical surgery, the surgical risk is assessed to be low if the walking distance is more than 25 shuttles (>400 m) during SWT or if the climbing altitude is higher than 22 m during SCT (Category IC).
CPET
CPET is a relatively complex physiologic evaluation technique that enables the real-time recording of ECG, exercise heart rate, minute ventilation, and oxygen intake per minute during exercise. CPET can yield the maximal oxygen consumption (VO2max), which is recommended by the previous guidelines as an important indicator for assessing cardiopulmonary function and surgical tolerance (9,33), allowing for even more accurate assessment of surgical risks. CPET is highly recommended by the guidelines issued by ERS/ESTS in 2009. For all patients scheduled for preoperative lung function tests, CPET is recommended for assessing surgical risk for patients with either FEV1 or DLCO less than 80% predicted. If CPET reveals that the VO2max is greater than 20 mL/kg/min or greater than 75% predicted, all the scheduled operations (including total pneumonectomy) can be performed. For patients with VO2max less than 20 mL/kg/min, PPO-FEV1 and PPO-DLCO need to be calculated according to resection size. If both of them are larger than 30% predicted, the planned resection can be performed; if either of them is less than 30% predicted, PPO-VO2max can be calculated according to resection size; and if PPO-VO2max is larger than 10 mL/kg/min or larger than 35% predicted, the scheduled resection can be performed. Studies have confirmed that the risk of postoperative death is closely related to VO2max, and patients with VO2max less than 10 mL/kg/min have extremely high postoperative mortality rate (3,11,34). A meta-analysis (including 14 studies, n=955) conducted by Benzo et al. (35) showed that VO2max decreased by 3 mL/kg/min in patients with postoperative complications compared with those without postoperative complications. Loewen et al. (36) found in 346 patients that VO2max <65% predicted was associated with more postoperative complications and a VO2max of <15 mL/kg/min prompted poorer long-term survival. These findings provided multicenter validation for the use of VO2max for preoperative assessment of lung cancer patients, and the authors encouraged an aggressive approach when evaluating these patients for surgery. Bayram et al. (37) did not find postoperative cardiopulmonary complications in patients with a VO2max of >15 mL/kg/min; in contrast, patients with VO2max <15 mL/kg/min had significantly increased postoperative lung complication rate and were at significantly increased risk for cardiovascular complications. Torchio et al. (38) investigated 145 COPD patients undergoing resection and found CPET is an important tool for assessing postoperative cardiopulmonary complications in these patients. They believed VO2max and V’E/V’CO2 are valuable indicators. Similarly, Bobbio et al. (39) concluded that VO2max measured during CPET was significantly correlated with the prognosis. Recently, Brunelli et al. (40) found in a study enrolled 200 patients that the postoperative mortality rate was 0% and the pulmonary complication rate was only 7% in patients with a VO2max of >20 mL/kg/min; in contrast, those with a VO2max of <12 mL/kg/min had 5-fold and 13-fold higher rates, respectively, of total cardiopulmonary complications and perioperative mortality rate.
Due to the presence of lung disease and long-term smoking, patients may have concurrent coronary atherosclerosis and cardiac insufficiency. CPET can also be used to evaluate and detect myocardial perfusion. Many guidelines recommend the use of CPET as a preoperative assessment tool for patients with a history of myocardial ischemia to assess and learn the patient’s myocardial perfusion reserves and cardiac function. Meanwhile, since CPET is a stress test, the patient should be assessed for cardiac function before CPET to avoid cardiovascular events induced during the test. The guidelines released by both American Heart Association/American College of Cardiology (41) and European Society of Cardiology/European Society of Anesthesiology (42) recommend the use of revised cardiac risk index (RCRI) (43) for assessing the operational risk in patients with cardiovascular diseases. Brunelli et al. (44) conducted a study in 1,696 patients undergoing lung resection and found four outcome variables were in the RCRI were reliably associated with major cardiac complications: cerebrovascular disease (1.5 points), cardiac ischemia (1.5 points), renal disease (creatinine >2 mg/dL or >176.8 µmol/L; 1 point), and pneumonectomy (1.5 points). Currently, these four variables have been used as thoracic RCRI (ThRCRI). Patients are scored according to their medical history and related tests before surgery. If ThRCRI is <2 points, no further cardiac assessment is required; pulmonary function assessment can be performed directly, and CPET can be carried out based on lung function. If ThRCRI is ≥2 points, the myocardial perfusion and cardiac function shall be assessed; if surgical intervention is required, no other assessment including CPET will be performed. The patients can be treated by the percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). A second assessment will be performed six weeks after the treatment before subsequent treatment is applied. In patients with a ThRCRI score of ≥2 points but without an indication for surgical intervention, CPET and lung function testing can be performed under drug treatment and other interventions.
In summary, CPET plays an important role in the assessment of operative risk in patients undergoing lung resection. CPET in nature is an exercise stress test that can simultaneously assess the cardiopulmonary functions; thus, it can evaluate both pulmonary complications and cardiovascular complications. Since CPET can effectively simulate the functioning of cardiopulmonary systems after surgery, it enables more accurate and intuitive assessments. However, CPET requires sophisticated equipment and professional testing personnel. At present, CPET is performed in only a few large clinical centers, and its values have not been fully recognized.
Recommendations
For all patients who may undergo radical surgery, patients can be categorized as a low-risk group if the VO2max is higher than 20 mL/kg/min or higher than 75% predicted during CPET; a surgery (e.g., total pneumonectomy) can be performed (Category IC).
For all patients who may undergo radical surgery, patients can be categorized as a high-risk group if the VO2max is lower than 10 mL/kg/min or lower than 35% predicted during CPET; treatment options should be carefully selected, including the re-calculation and assessment of the resection size and the use of non-surgical treatments (Category IC).
For all patients who may undergo radical surgery, the PPO-VO2max should be calculated based on resection size in patients with a VO2max of 10–20 mL/kg/min or 35–75% predicted during CPET. If the calculated PPO-VO2max is higher than 10 mL/kg/min or larger than 35% predicted, a surgery performed within the calculated extent of resection is recommended; however, the operational risk is still high in these patients (Category IC).
Arterial blood gas (ABG) analysis
ABG analysis has previously been regarded as an important preoperative evaluation method. In particular, when pulmonary diffusion capacity testing is not available, ABG analysis combined with spirometry can, to some extent, evaluates the diffusion capacity of lungs. Hypercapnia (PaCO2 >45 mmHg) has long been considered a relative surgical contraindication as it might have been associated with increased incidences of postoperative adverse events (45,46). However, new studies have shown that hypercapnia is not an independent perioperative risk factor for perioperative complications or death associated with lung resection. Stein et al. (47) found no correlation between PaCO2 >45 mmHg and postoperative mortality. Two other studies have also demonstrated that patients with hypercapnia, as indicated by ABG analysis, showed no significant increase in perioperative complication rates compared with otherwise normal patients (48,49). In another study, the incidence of perioperative complications significantly increased in patients with preoperative hypoxemia (50). Furthermore, ABG analysis may reflect the diffusion capacity of lung tissues. For clinical centers that have difficulty in measuring DLCO, ABG analysis combined with imaging scans can be used to evaluate some low-risk patients. If blood gas analysis yields normal results, chest CT reveals that there is no visible lesion in lung tissue except the resected part and the lung texture is even and normal, and the clinical measurement of DLCO is difficult, the combined method can be applied for assessing diffusion capacity. However, for patients with abnormal blood gas profile or imaging findings, measurement of DLCO is recommended for assessing the diffuse capacity.
Preoperative pulmonary function tests in patients with specific conditions
Preoperative pulmonary assessment in patients undergoing surgery for simultaneous bilateral lung cancer
The diagnosis rate of primary simultaneous bilateral lung cancer has increased with the wider application of CT examination and thus, the earlier diagnosis of lung cancer. However, the preoperative pulmonary function tests and the decision-making on resection sizes in such patients can be challenging (51,52). Many studies have demonstrated the feasibility of surgical therapy for bilateral multiple primary lung cancer (53-57). Some recent articles have reported the radical surgery for simultaneous bilateral lung cancer (58-60), prompting the possibility of treating this malignancy with simultaneous bilateral surgery. However, compared with the unilateral surgeries, the bilateral surgeries are associated with a larger loss of lung function within a short period and higher perioperative complication rate due to the simultaneously damaged integrity of bilateral thoracic cavity and the lack of compensation by a healthy side. For bilateral sub lobectomy or unilateral lobectomy combined with contralateral sub lobectomy, the preoperative assessment can follow the pulmonary assessment methods used for unilateral surgery. However, the preoperative pulmonary assessment for simultaneous bilateral major lung resection remains controversial. Our study on patients undergoing unilateral lobectomy combined with resection of two or more contralateral segments has shown that the incidences of perioperative complications in these patients were significantly higher than those in patients receiving a unilateral surgery. The results suggest the preoperative pulmonary assessment for bilateral pneumonectomy should be performed with caution due to the simultaneous bilateral destruction of the integrity of the thoracic cavity and the lack of contralateral compensation. However, limited by its small sample size, our study only confirmed that the cut-off values needed to be further increased during pulmonary assessment for a simultaneous bilateral surgery, whereas the specific cut-off values need to be further investigated in studies with large sample sizes.
Preoperative pulmonary assessment in patients with chronic obstructive pulmonary disease (COPD) and/or other diffuse lung diseases
The difference in lung function between patients with COPD and otherwise, healthy patients needs to be further clarified as lung volume reduction may occur after lung resection. Brunelli et al. (61) found that COPD patients with a PPO-FEV1 of <40% predicted had a perioperative mortality rate of only 4.8% after lobectomy. Linden et al. (62) investigated the outcomes of patients with preoperative FEV1 <35% predicted (mean: <26% predicted) and concluded FEV1 alone could not adequately predict perioperative complications and deaths. Thus, the accuracy of FEV1 alone in preoperative pulmonary evaluation has been questioned. In lung cancer patients with moderate to severe COPD, the postoperative lung function loss can be smaller than predicted (and even increase in some cases) due to the effect of lung volume reduction if the resected lung parenchyma is also the main diseased area of COPD (63-65). These studies also showed that the lung volume reduction effect in these patients occurred immediately after surgery, suggesting the effect of radical surgery for lung cancer on lung function is smaller in these patients than in a patient without COPD. Thus, less strict assessment criteria may be applied in these patients, along with the use of multiple testing methods for a more detailed and accurate assessment. Notably, preoperative preparation of the respiratory system (e.g., clearing the respiratory tract with phlegm-resolving drugs and encouraging patients to perform physical exercises such as stair climbing) in COPD patients can remarkably improve lung function. In these patients, a second assessment can be performed after the preparation to ensure the patient has achieved the required lung function criteria before surgery.
For preoperative pulmonary tests in patients with diffuse lung disease (e.g., diffuse bullae or interstitial lung disease), the assessment methods are theoretically similar to those for ordinary patients since these patients have no clear lesions or non-functional areas. However, certain adjustments need to be made in preoperative pulmonary tests, depending on the different characteristics of the disease. In COPD patients, since the lesions in two upper lobes are often larger than those in two lower lobes, the indication for resection of the upper lobes can be set wider; in contrast, the resection of the lower lobes should be more cautious. Interstitial lung disease is a group of clinicopathological entities with diffused pulmonary parenchyma, alveolar inflammation, and interstitial fibrosis as basic lesions and with active dyspnea, diffuse imaging changes, restrictive ventilatory dysfunction, decreased diffusing capacity, and hypoxemia as clinical manifestations. Preoperative pulmonary tests in patients with idiopathic pulmonary fibrosis (IPF) should be particularly cautious. The abnormal lung function in IPF patients is featured by the marked decrease in forced vital capacity (FVC) and DLCO, whereas the decrease in FEV1 is not obvious. Research has confirmed that FVC is an independent risk factor during the surgical evaluation of such patients (66). FEV1 is often used as a clinical assessment indicator for IPF patients, which often results in overestimated lung function; however, there is no widely-recognized cut-off value of FVC for the pre-operative pulmonary assessment in IPF patients. IPF Patients may suffer from rapid disease progression after surgery; for these patients, the pulmonary assessment should be based on FVC and DLCO as well as the functional and structural changes of the resected and preserved lung tissues. Preoperative pulmonary assessment should be stricter for patients undergoing upper lobe resection because patients with interstitial lung disease tend to have more severe lesions in lower lobes. Notably, for patients with idiopathic fibrosis, even if their preoperative pulmonary ventilation function and diffusion capacity meet the surgical requirements, patients and their families should be informed of the risk of death due to acute exacerbation of pulmonary interstitial lesions.
Pulmonary ventilation/perfusion imaging can be performed with the support of related equipment. However, the results provided by this technique are not three-dimensional. The functional areas of different lung lobes have obvious overlap and cannot be separated. Since the lung function loss of patients undergoing upper lobe resection is often overestimated, the results of this test are just suggestive.
Preoperative pulmonary assessment in patients with a localized lesion or non-functioning lung tissue
Some benign lung diseases are risk factors for lung cancer, and lung cancer often occurs in the region of these underlying lesions such as COPD, interstitial lung disease, and tuberculosis (67). Also, tumors originating in the proximal bronchus can cause luminal obstruction, leading to post-obstructive pneumonia or atelectasis in the distal lung tissue. Benign diseases may be confined to a region where lung function is generally poor. The presence of an area with localized lesions or a non-functioning area results in the uneven distribution of functioning lung units. Thus, the preoperative pulmonary assessment should consider whether the surgical resection site includes such an area. A non-functioning area should be defined as tissues that cannot complete normal blood-gas exchange. These areas can be determined by pulmonary ventilation/perfusion scan, which can assess the level of ventilation/perfusion in each lung tissue area; specially, anterior-posterior overlap exists in lung tissue imaging, which may lead to over- or under-estimation of postoperative lung function (68). For lung cancer patients with impaired lung function, removal of tumor-containing lung tissues in the non-functioning area did not increase the risk of postoperative complications such as respiratory failure. Resection of non-functioning areas can increase the proportion of blood flow in the ventilated lung area. The cut-off values used in preoperative pulmonary assessment should be set wider for these patients. For patients undergoing only the resection of functioning lung units but not the removal of non-functioning areas, the preoperative pulmonary assessment should be more cautious. In patients having areas with impaired lung function, the lung function of the resected part is remarkably worse than that of normal lung units; thus, the preoperative pulmonary assessment often results in underestimated lung function. Single-photon emission computed tomography (SPECT) enables more accurate lung function assessment as SPECT can calculate the functional status in a specific lung area without being affected by spatial overlap.
Pulmonary assessment before a second lung surgery
The preoperative pulmonary assessment before a second surgery is more complicated because the redistribution of blood flow and ventilation after the first operation results in the altered distribution of the function of each lobe. The ipsilateral and contralateral reoperation should be tailored following preoperative findings. If necessary, the pulmonary ventilation/perfusion scan can be performed to identify the functional proportions of the lateral and various parts, to determine the proportions of functional lung tissues removed. Subsequently, the corresponding formulas are used to calculate PPO-FEV1 and PPO-DLCO, and the results are substituted into the path to evaluate the patient’s surgical risk. Meanwhile, the following conditions should also be considered during preoperative evaluation.
Factors affecting lung function in a second lung surgery include:
- Whether the two operations are performed ipsilaterally or contralaterally: if the second surgery is performed ipsilaterally, the reduction in postoperative pulmonary function is relatively small; if the second surgery is performed contralaterally, it can have a more severe impact on lung function and is more likely to induce respiratory failure.
- The interval between two operations: if the interval between two operations exceeds six months, the decreased lung function after the first operation has almost completely recovered; if the interval is within six months, the decreased lung function following the first operation can be further restored.
- Calculation of PPO-FEV1 and PPO-DLCO: after the first operation, the residual pulmonary function at the operated side may be greatly affected due to factors including the shrunk thoracic cage on the operated side and the muscle damage on the chest wall. It is not advisable to calculate the PPO-FEV1 and PPO-DLCO based directly on the number of lung segments. The postoperative residual lung function can be assessed by calculating the ventilation/perfusion ratio and lung ventilation function (Figure 1).
Supplementary
Calculation of PPO-FEV1, PPO-DLCO, and PPO-VO2max
Calculation of PPO-FEV1
For patients undergoing total pneumonectomy: PPO FEV1 = preoperative FEV 1 × (1 − a fraction of the total perfusion for the resected lung);
For patients undergoing lobectomy: PPO FEV1 = preoperative FEV 1 × (1 − y/z).
Calculation of PPO-DLCO
For patients undergoing total pneumonectomy: PPO-DLCO = preoperative DLCO × (1 − a fraction of the total perfusion for the resected lung);
For patients undergoing lobectomy: PPO-DLCO = preoperative DLCO × (1 − y/z).
Calculation of PPO-VO2max
For patients undergoing total pneumonectomy: PPO-VO2max = preoperative VO2max × (1 − a fraction of the total perfusion for the resected lung);
For patients undergoing lobectomy: PPO-VO2max = preoperative VO2max × (1 − y/z).
Note: y is the number of functional or unobstructed lung segments to be removed, and z is the total number of functional segments.
Summary of recommendations for patients scheduled for a radical surgery
- Measure both FEV1 and DLCO and calculate and evaluate PPO-FEV1 and PPO-DLCO according to the resection sizes (Category IB).
- No further evaluation is required if the PPO-FEV1 and PPO-DLCO are greater than 60% predicted according to the resection size, and surgical resection can be scheduled (Category IC).
- Low-technology exercise tests (including SCT and SWT) are recommended if either PPO-FEV1 or PPO-DLCO is less than 60% predicted and both are greater than 30% predicted according to the resection size (Category IC).
- Cardiopulmonary exercise test (CPET) is recommended to measure VO2max if either PPO-FEV1 or PPO-DLCO is less than 30% predicted according to the resection size (Category IB).
- Cardiopulmonary exercise test (CPET) is recommended to measure VO2max if the walking distance is less than 25 shuttles (<400 m) in SWT or if the climbing altitude is below 22 m during SCT (Category IC).
- Patients can be categorized as a low-risk group if the VO2max is higher than 20 mL/kg/min or larger than 75% predicted during CPET; a surgery (e.g., total pneumonectomy) can be performed (Category IC).
- Patients can be categorized as a high-risk group if the VO2max is lower than 10mL/kg/min or lower than 35% predicted during CPET; treatment options should be carefully selected, including the re-calculation and assessment of the resection size and the use of non-surgical treatments (Category IC).
The PPO-VO2max should be calculated based on resection size in patients with a VO2max of 10–20 mL/kg/min or 35–75% predicted during CPET; if the calculated PPO-VO2max is higher than 10 mL/kg/min or larger than 35% predicted, a surgery performed within the calculated scope is recommended; however, the surgical risk is still high in these patients (Category IC).
Acknowledgments
Funding: None.
Footnote
Conflicts of Interest: Dr. Gening Jiang serves as an unpaid Editor-in-Chief of Current Challenges in Thoracic Surgery. Dr. Chang Chen serves as an unpaid Associate Editor-in-Chief of Current Challenges in Thoracic Surgery. Drs Haifeng Wang and Yang Yang serve as unpaid Managing Editors of Current Challenges in Thoracic Surgery. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Boushy SF, Billig DM, Noml LB, et al. Clinical course related to preoperative and postoperative pulmonary function in patients with bronchogenic carcinoma. Chest 1971;59:383-91. [Crossref] [PubMed]
- Miller JI. Physiologic evaluation of pulmonary function in the candidate for lung resection. J Thorac Cardiovasc Surg 1993;105:347-51; discussion 351-2. [PubMed]
- Bechard D, Wbtstein L. Assessment of exercise oxygen consumption as preoperative criterion for lung resection. Ann Thorac Surg 1987;44:344-9. [Crossref] [PubMed]
- Ferguson MK, Little L, Rizzo L, et al. Diffusing capacity predicts morbidity and mortality after pulmonary resection. J Thorac Cardiovasc Surg 1988;96:894-900. [PubMed]
- Wyser C, Stulz P, Solèr M, et al. Prospective evaluation of algorithm for the functional assessment of lung resection candidates. Am J Respir Crit Care Med 1999;159:1450-6. [Crossref] [PubMed]
- BTS guidelines: guidelines on the selection of patients with lung cancer for surgery. Thorax 2001;56:89-108. [Crossref] [PubMed]
- Brunelli A, Charloux A, Bolliger CT, et al. ERS/ESTS clinical guidelines on fitness for radical therapy in lung cancer patients (surgery and chemo-radiotherapy). Eur Respir J 2009;34:17-41. [Crossref] [PubMed]
- Brunelli A, Kim AW, Berger KI, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013;143:e166S-90S.
- Colice GL, Shafazand S, Griffin JP, et al. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: ACCP evidenced-based clinical practice guidelines (2nd edition). Chest 2007;132:161S-77S.
- Bolliger CT, Jordan P, Soler M, et al. Exercise capacity as a predictor of postoperative complications in lung resection candidates. Am J Respir Crit Care Med 1995;151:1472-80. [Crossref] [PubMed]
- Holden DA, Rice TW, Stelmach K, et al. Exercise testing, 6-min walk, and stair climb in the evaluation of patients at high risk for pulmonary resection. Chest 1992;102:1774-9. [Crossref] [PubMed]
- Markos J, Mullan BP, Hillman DR, et al. Preoperative assessment as a predictor of mortality and morbidity after lung resection. Am Rev Respir Dis 1989;139:902-10. [Crossref] [PubMed]
- Pierce RJ, Copland JM, Sharpe K, et al. Preoperative risk evaluation for lung cancer resection: predicted postoperative product as a predictor of surgical mortality. Am J Respir Crit Care Med 1994;150:947-55. [Crossref] [PubMed]
- Wahi R, McMurtrey MJ, DeCaro LF, et al. Determinants of perioperative morbidity and mortality after pneumonectomy. Ann Thorac Surg 1989;48:33-7. [Crossref] [PubMed]
- Nakahara K, Monden Y, Ohno K, et al. A method for predicting postoperative lung function and its relation to postoperative complications in patients with lung cancer. Ann Thorac Surg 1985;39:260-5. [Crossref] [PubMed]
- Ferguson MK, Siddique J, Karrison T. Modeling major lung resection outcomes using classifi cation trees and multiple imputation techniques. Eur J Cardiothorac Surg 2008;34:1085-9. [Crossref] [PubMed]
- Licker MJ, Widikker I, Robert J, et al. Operative mortality and respiratory complications after lung resection for cancer: impact of chronic obstructive pulmonary disease and time trends. Ann Thorac Surg 2006;81:1830-7. [Crossref] [PubMed]
- Berry MF, Villamizar-Ortiz N, Tong BC, et al. Pulmonary function tests do not predict pulmonary complications after thoracoscopic lobectomy. Ann Thorac Surg 2010;89:1044-51. [Crossref] [PubMed]
- Cerfolio RJ, Bryant AS. Different diffusing capacity of the lung for carbon monoxide as predictors of respiratory morbidity. Ann Thorac Surg 2009;88:405-10. [Crossref] [PubMed]
- Liptay MJ, Basu S, Hoaglin M, et al. Diffusion lung capacity for carbon monoxide (DLCO) is an independent prognostic factor for long-term survival after curative lung resection for cancer. J Surg Oncol 2009;100:703-7. [Crossref] [PubMed]
- Ferguson MK, Dignam JJ, Siddique J, et al. Diffusing capacity predicts long-term survival after lung resection for cancer. Eur J Cardiothorac Surg 2012;41:e81-6. [Crossref] [PubMed]
- Kobayashi K, Saeki Y, Kitazawa S, et al. Three-dimensional computed tomographic volumetry precisely predicts the postoperative pulmonary function. Surg Today 2017;47:1303-11. [Crossref] [PubMed]
- Le Roux PY, Leong TL, Barnett SA, et al. Gallium-68 perfusion positron emission tomography/computed tomography to assess pulmonary function in lung cancer patients undergoing surgery. Cancer Imaging 2016;16:24-32. [Crossref] [PubMed]
- Bolton JW, Weiman DS, Haynes JL, et al. Stair climbing as an indicator of pulmonary function. Chest 1987;92:783-8. [Crossref] [PubMed]
- Brunelli A, Al Refai M, Monteverde M, et al. Stair climbing test predicts cardiopulmonary complications after lung resection. Chest 2002;121:1106-10. [Crossref] [PubMed]
- Brunelli A, Refai M, Xiumé F, et al. Performance at symptom limited stair-climbing test is associated with increased cardiopulmonary complications, mortality, and costs after major lung resection. Ann Thorac Surg 2008;86:240-7. [Crossref] [PubMed]
- Brunelli A, Xiumé F, Refai M, et al. Peak oxygen consumption measured during the stair-climbing test in lung resection candidates. Respiration 2010;80:207-11. [Crossref] [PubMed]
- Novoa NM, Esteban P, Podrigurez M, et al. Functional evaluation before lung resection: searching for a low technology test in a safer environment for the patient: a pilot study. Eur J Cardiothorac Surg 2017;51:856-60. [Crossref] [PubMed]
- Singh SJ, Morgan MDL, Scott S, et al. Development of a shuttle walking test of disability in patients with chronic airways obstruction. Thorax 1992;47:1019-24. [Crossref] [PubMed]
- Win T, Jackson A, Groves AM, et al. Comparison of shuttle walk with measured peak oxygen consumption in patients with operable lung cancer. Thorax 2006;61:57-60. [Crossref] [PubMed]
- Benzo RP, Sciurba FC. Oxygen consumption, shuttle walking test and the evaluation of lung resection. Respiration 2010;80:19-23. [Crossref] [PubMed]
- Nakagawa T, Tomioka Y, Toyazaki T, et al. Association between values of preoperative 6-min walk test and surgical outcomes in lung cancer Patients with decreased predicted postoperative pulmonary function. Gen Thorac Cardiovasc Surg 2018;66:220-4. [Crossref] [PubMed]
- Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax 2010;65:iii1-27. [Crossref] [PubMed]
- Olsen GN, Weiman DS, Bolton JWR, et al. Submaximal invasive exercise testing and quantitative lung scanning in the evaluation for tolerance of lung resection. Chest 1989;95:267-73. [Crossref] [PubMed]
- Benzo R, Kelley GA, Recchi L, et al. Complications of lung resection and exercise capacity: a meta-analysis. Respir Med 2007;101:1790-7. [Crossref] [PubMed]
- Loewen GM, Watson D, Kohman L, et al. Cancer and Leukemia Group B Preoperative exercise Vo2 measurement for lung resection candidates: results of Cancer and Leukemia Group B Protocol 9238. J Thorac Oncol 2007;2:619-25. [Crossref] [PubMed]
- Bayram AS, Candan T, Gebitekin C. Preoperative maximal exercise oxygen consumption test predicts postoperative pulmonary morbidity following major lung resection. Respirology 2007;12:505-10. [Crossref] [PubMed]
- Torchio R, Guglielmo M, Giardino R, et al. Exercise ventilatory inefficiency and mortality in patients with chronic obstructive pulmonary disease undergoing surgery for nonsmall-cell lung cancer. Eur J Cardiothorac Surg 2010;38:14-9. [Crossref] [PubMed]
- Bobbio A, Chetta A, Internullo E, et al. Exercise capacity assessment in patients undergoing lung resection. Eur J Cardiothorac Surg 2009;35:419-22. [Crossref] [PubMed]
- Brunelli A, Belardinelli R, Refai M, et al. Peak oxygen consumption during cardiopulmonary exercise test improves risk stratification in candidates to major lung resection. Chest 2009;135:1260-7. [Crossref] [PubMed]
- Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation 2007;116:e418-99. [PubMed]
- Poldermans D, Bax JJ, Boersma E, et al. Task Force for Preoperative Cardiac Risk Assessment and Perioperative Cardiac Management in Non-cardiac Surgery; European Society of Cardiology (ESC). Guidelines for pre-operative cardiac risk assessment and perioperative cardiac management in non-cardiac surgery. Eur Heart J 2009;30:2769-812. [Crossref] [PubMed]
- Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100:1043-9. [Crossref] [PubMed]
- Brunelli A, Varela G, Salati M, et al. Recalibration of the revised cardiac risk index in lung resection candidates. Ann Thorac Surg 2010;90:199-203. [Crossref] [PubMed]
- Celli BR. What is the value of preoperative pulmonary function testing? Med Clin North Am 1993;77:309-25. [Crossref] [PubMed]
- Zibrak JD, O’Donnell CR, Marton K. Indications for pulmonary function testing. Ann Intern Med 1990;112:763-71. [Crossref] [PubMed]
- Stein M, Koota GM, Simon M, et al. Pulmonary evaluation of surgical patients. JAMA 1962;181:765-70. [Crossref] [PubMed]
- Kearney DJ, Lee TH, Reilly JJ, et al. Assessment of operative risk in patients undergoing lung resection. Importance of predicted pulmonary function. Chest 1994;105:753-9. [Crossref] [PubMed]
- Harpole DH, Liptay MJ, DeCamp MM Jr, et al. Prospective analysis of pneumonectomy: risk factors for major morbidity and cardiac dysrhythmias. Ann Thorac Surg 1996;61:977-82. [Crossref] [PubMed]
- Turner SE, Eastwood PR, Cecins NM, et al. Physiologic responses to incremental and self-paced exercise in COPD: a comparison of three tests. Chest 2004;126:766-73. [Crossref] [PubMed]
- Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 2011;365:395-409. [Crossref] [PubMed]
- Shah AA, Barfield ME, Kelsey CR, et al. Outcomes after surgical management of synchronous bilateral primary lung cancers. Ann Thorac Surg 2012;93:1055-60. [Crossref] [PubMed]
- Nakata M, Sawada S, Yamashita M, et al. Surgical treatments for multiple primary adenocarcinoma of the lung. Ann Thorac Surg 2004;78:1194-9. [Crossref] [PubMed]
- De Leyn P, Moons J, Vansteenkiste J, et al. Survival after resection of synchronous bilateral lung cancer. Eur J Cardiothorac Surg 2008;34:1215-22. [Crossref] [PubMed]
- Adebonojo SA, Moritz DM, Danby CA. The results of modern surgical therapy for multiple primary lung cancers. Chest 1997;112:693-701. [Crossref] [PubMed]
- Deschamps C, Pairolero PC, Trastek VF, et al. Multiple primary lung cancers. Results of surgical treatment. J Thorac Cardiovasc Surg 1990;99:769-77. [PubMed]
- Tsunezuka Y, Matsumoto I, Tamura M, et al. The results of therapy for bilateral multiple primary lung cancers: 30 years experience in a single centre. Eur J Surg Oncol 2004;30:781-5. [Crossref] [PubMed]
- Matsubara T, Toyokawa G, Kinoshita F, et al. Safety of simultaneous bilateral pulmonary resection for metastatic lung tumors. Anticancer Res 2018;38:1715-9. [PubMed]
- Yao F, Yang H, Zhao H. Single-stage bilateral pulmonary resections by video-assisted thoracic surgery for multiple small nodules. J Thorac Dis 2016;8:469-75. [Crossref] [PubMed]
- Makino T, Hata Y, Otsuka H, et al. Simultaneous resection of bilateral anomalous systemic supply to the basal segments of the lungs: a case report. J Cardiothorac Surg 2015;10:140. [Crossref] [PubMed]
- Brunelli A, Al Refai M, Monteverde M, et al. Predictors of early morbidity after major lung resection in patients with and without airflow limitation. Ann Thorac Surg 2002;74:999-1003. [Crossref] [PubMed]
- Linden PA, Bueno R, Colson YL, et al. Lung resection in patients with preoperative FEV1 <35% predicted. Chest 2005;127:1984-90. [Crossref] [PubMed]
- Brunelli A, Xiumé F, Refai M, et al. Evaluation of expiratory volume, diffusion capacity, and exercise tolerance following major lung resection: a prospective follow-up analysis. Chest 2007;131:141-7. [Crossref] [PubMed]
- Brunelli A, Refai M, Salati M, et al. Predicted versus observed FEV1 and DLCO after major lung resection: a prospective evaluation at different postoperative periods. Ann Thorac Surg 2007;83:1134-9. [Crossref] [PubMed]
- Baldi S, Ruffini E, Harari S, et al. Does lobectomy for lung cancer in patients with chronic obstructive pulmonary disease affect lung function? A multicenter national study. J Thorac Cardiovasc Surg 2005;130:1616-22. [Crossref] [PubMed]
- Omori T, Tajiri M, Baba T, et al. Pulmonary resection for lung cancer in patients with idiopathic interstitial pneumonia. Ann Thorac Surg 2015;100:954-60. [Crossref] [PubMed]
- Brenner DR, Boffetta P, Duell EJ, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol 2012;176:573-85. [Crossref] [PubMed]
- Suga K, Tsukuda T, Awaya H, et al. Interactions of regional respiratory mechanics and pulmonary ventilatory impairment in pulmonary emphysema: assessment with dynamic MRI and xenon-133 single-photon emission CT. Chest 2000;117:1646-55. [Crossref] [PubMed]
Cite this article as: Jiang G, Zhang L, Zhu Y, Chen C, Zhou X, Liu J, Zhang P, Wang H, Xie B, Wang H, Jiang L, Fan J, Zhao D, Chen Q, Duan L, He W, Zhou Y, Liu H, Zhao X, Qin X, Yang Y, Ning Y, Xie D, Dai J. Clinical consensus on preoperative pulmonary function assessment in patients undergoing pulmonary resection (first edition). Curr Chall Thorac Surg 2019;1:7.